(1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol Dihydrochloride
Abstract
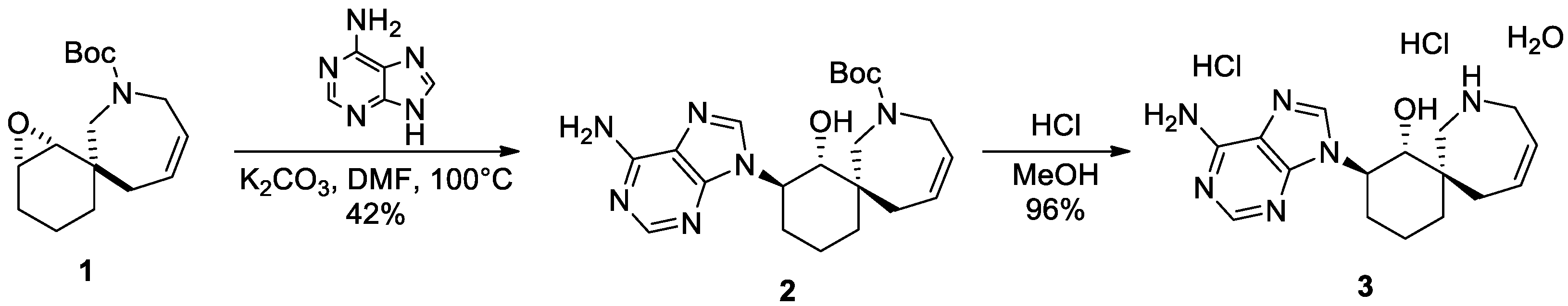
1. Introduction
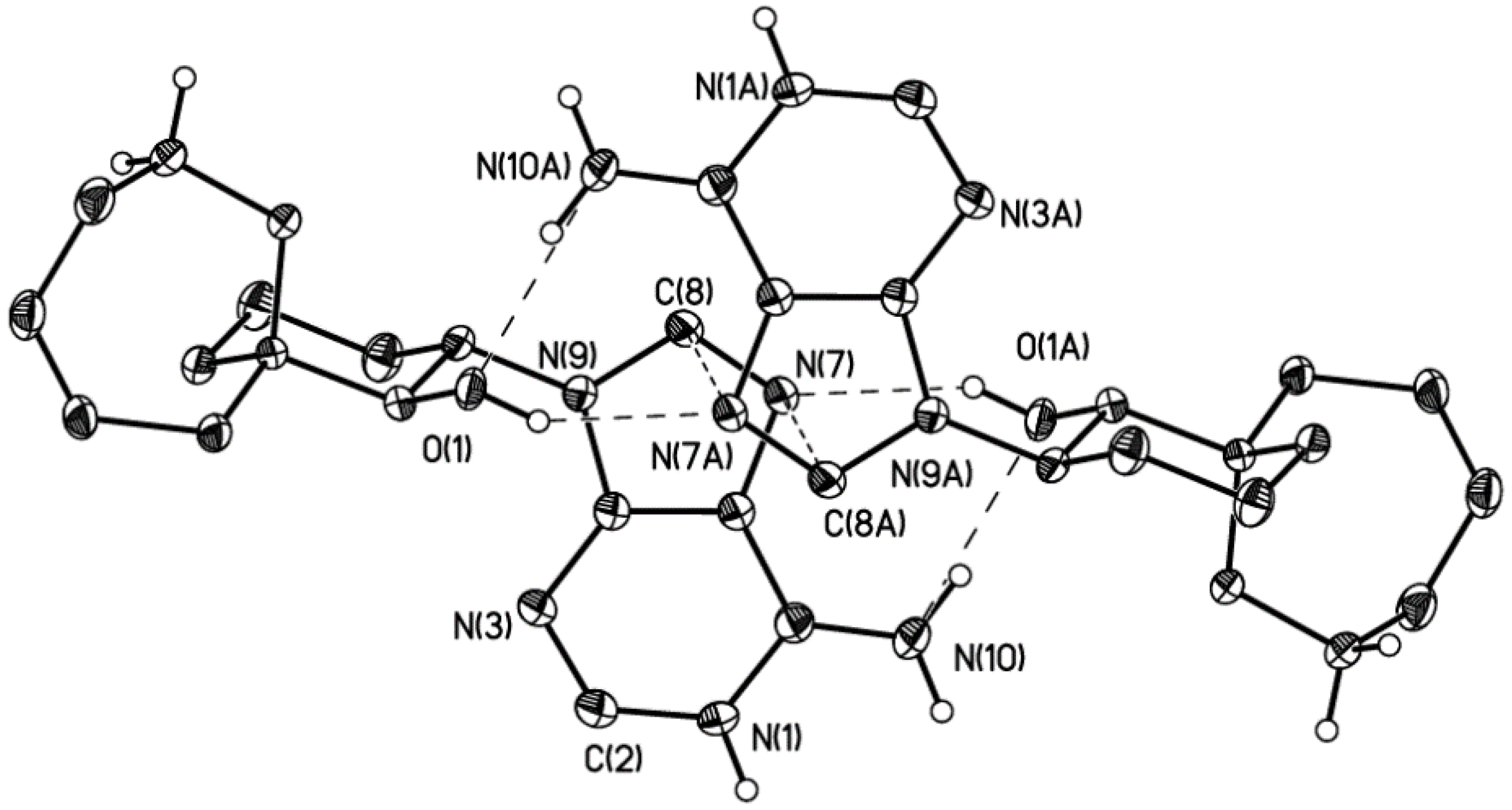
2. Results
3. Materials and Methods
3.1. General
3.2. Synthesis of Tert-butyl (1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-1-hydroxy-8-azaspiro[5.6]dodec-10-ene-8-carboxylate (2)
3.3. Synthesis of Dihydrochloride of (1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol (3)
3.4. Crystallography Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, I.B.; Macdonald, S.J.F.; Procopiou, P.A. Medicinal chemistry in drug discovery in big pharma: Past, present and future. Drug Discov. 2018, 23, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.-V.T.; Bode, J.W. Synthesis of Saturated N-Heterocycles. J. Org. Chem. 2014, 79, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-J.; Tice, C.M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 831–834. [Google Scholar] [CrossRef]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Wanner, J.W.; Koomen, G.J. Studies in Natural Products Chemistry: Stereoselectivity in Synthesis and Biosynthesis of Lupine and Nitraria Alkaloids; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 1994; p. 731. [Google Scholar]
- Boubaker, J.; Bhouri, W.; Sghaier, B.M.; Bouhlel, I.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Leaf extracts from Nitraria retusa promote cell population growth of human cancer cells by inducing apoptosis. Cancer Cell Int. 2011, 11, 37. [Google Scholar] [CrossRef]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.-Y.; Zhou, J. Therapeutic Potential of Spirooxindoles as Antiviral Agents. ACS Infect Dis. 2016, 10, 382–392. [Google Scholar] [CrossRef]
- Liebowitz, S.M.; Belair, E.J.; Witiak, D.T.; Lednicer, D. Synthesis and cardiovascular activity of a series of spiroureas. Eur. J. Med. Chem. 1986, 21, 439–444. [Google Scholar]
- Yang, J.; Park, Y.; Yang, S.; Lee, G.; Ha, M.W.; Kim, M.-H.; Hong, S.; Park, H.-G. Enantioselective Total Synthesis of Nitraria Alkaloids: (+)-Nitramine, (+)-Isonitramine, (−)-Isonitramine, and (−)-Sibirine via Asymmetric Phase-Transfer Catalytic α-Allylations of α-Carboxylactams. J. Org. Chem. 2021, 86, 4375–4390. [Google Scholar] [CrossRef]
- Altar, C.A.; Marien, M.R. [3H]vesamicol binding in brain: Autoradiographic distribution, pharmacology, and effects of cholinergic lesions. Synapse 1988, 2, 486–493. [Google Scholar] [CrossRef]
- Marshall, G.; Parsons, S.M. The vesicular acetylcholine transport system. Trends Neurosci. 1987, 10, 174–177. [Google Scholar] [CrossRef]
- Bajracharya, G.B.; Arai, M.A.; Koranne, P.S.; Suzuki, T.; Takizawa, S.; Sasa, H. Development of Chiral Spiro Ligands for Metal-Catalyzed Asymmetric Reactions. Bull. Chem. Soc. Jpn. 2009, 82, 285–302. [Google Scholar] [CrossRef]
- Bajracharya, G.B. Design and Synthesis of Chiral Spiro Ligands. J. Nepal Chem. Soc. 2013, 28, 1–23. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F. Escape from flatland 2: Complexity and promiscuity. ChemMedComm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Iusupov, I.R.; Lukyanenko, E.R.; Altieri, A.; Kurkin, A.V. Design and Synthesis of Fsp3-enriched Spirocyclic-Based Biological Screening Compound Arrays via DOS strategies and their NNMT Inhibition Profiling. ChemMedChem. 2022. [Google Scholar] [CrossRef]
- Castro, S.; Camarasa, M.-J. Polypharmacology in HIV inhibition: Can a drug with simultaneous action against two relevant targets be an alternative to combination therapy? Eur. J. Med. Chem. 2018, 150, 206–227. [Google Scholar] [CrossRef]
- Belov, D.S.; Lukyanenko, E.R.; Kurkin, A.V.; Yurovskaya, M.A. Highly Stereoselective and Scalable Synthesis of trans-Fused Octahydrocyclohepta[b]pyrrol-4(1H)-ones via the Aza-Cope–Mannich Rearrangement in Racemic and Enantiopure Forms. J. Org. Chem. 2012, 77, 10125–10134. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- SADABS, version 2008-1; Bruker AXS: Madison, WI, USA, 2008.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iusupov, I.R.; Lyssenko, K.A.; Altieri, A.; Kurkin, A.V. (1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol Dihydrochloride. Molbank 2022, 2022, M1495. https://doi.org/10.3390/M1495
Iusupov IR, Lyssenko KA, Altieri A, Kurkin AV. (1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol Dihydrochloride. Molbank. 2022; 2022(4):M1495. https://doi.org/10.3390/M1495
Chicago/Turabian StyleIusupov, Ildar R., Konstantin A. Lyssenko, Andrea Altieri, and Alexander V. Kurkin. 2022. "(1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol Dihydrochloride" Molbank 2022, no. 4: M1495. https://doi.org/10.3390/M1495
APA StyleIusupov, I. R., Lyssenko, K. A., Altieri, A., & Kurkin, A. V. (2022). (1RS,2RS,6RS)-2-(6-Amino-9H-purin-9-yl)-8-azaspiro[5.6]dodec-10-en-1-ol Dihydrochloride. Molbank, 2022(4), M1495. https://doi.org/10.3390/M1495





