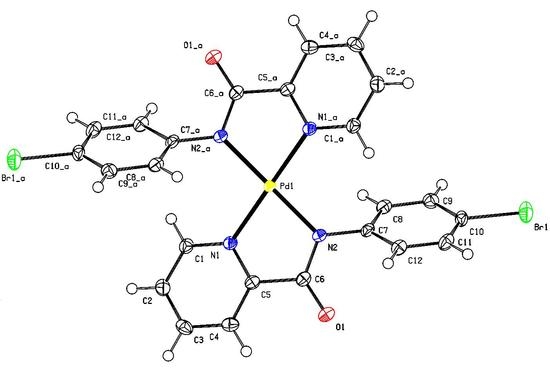
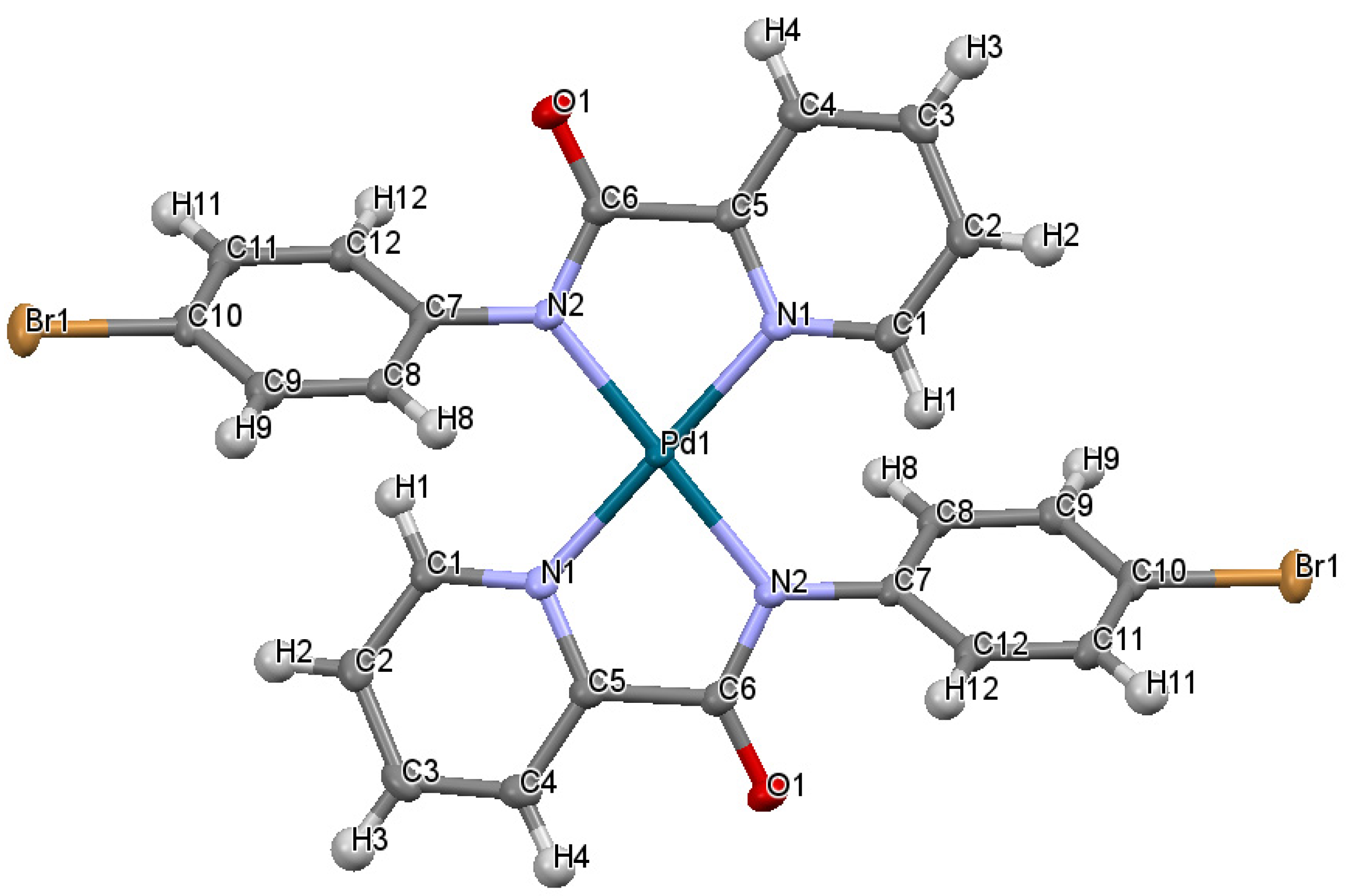
bis[N-(4-Bromophenyl)pyridine-2-carboxamidato]palladium
Abstract
1. Introduction
2. Results
3. Materials and Methods
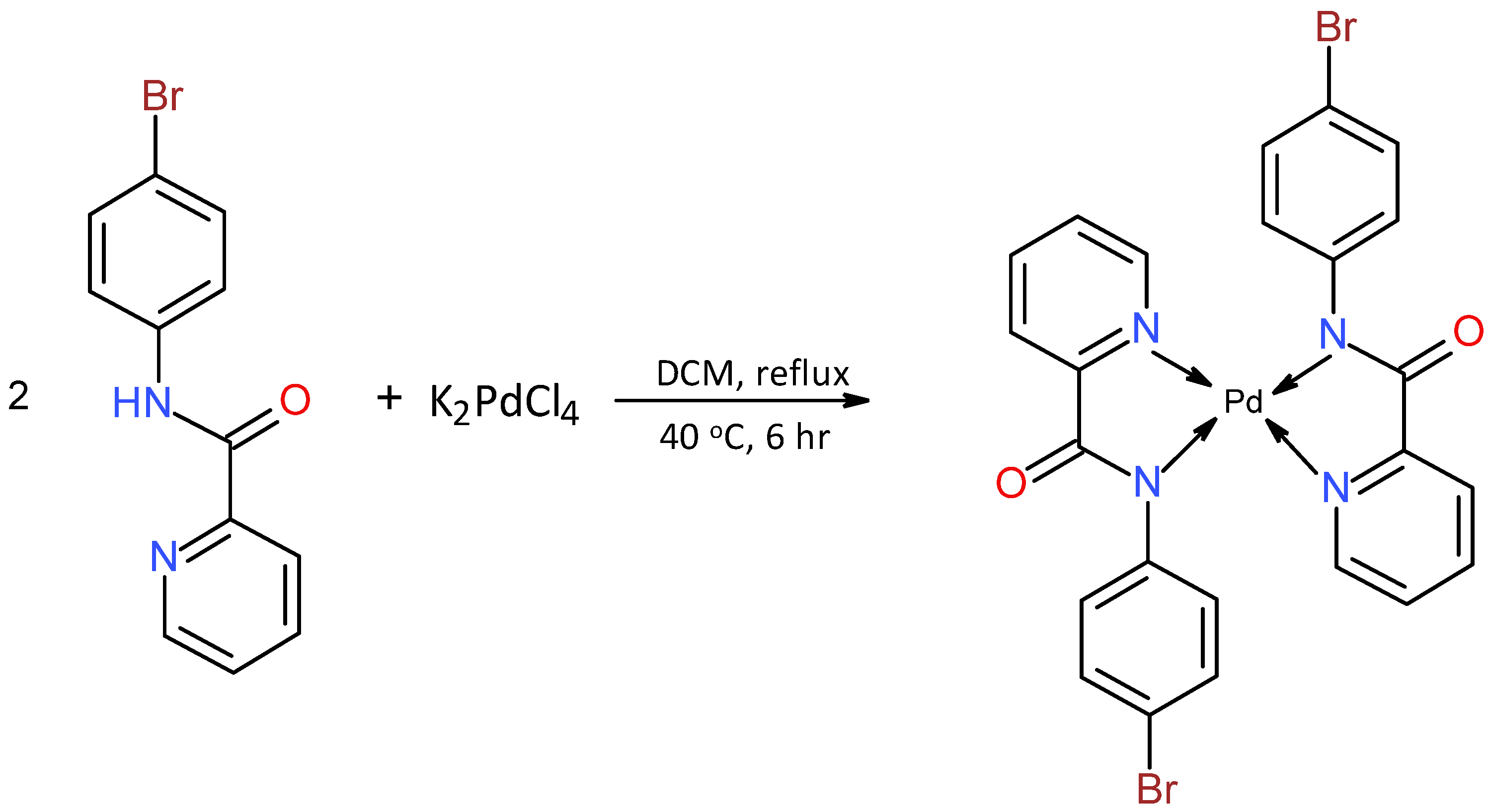
3.1. Synthesis of bis[N-(4-bromophenyl)pyridine-2-carboxamidato]Palladium (C1)
3.2. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, B.; Vancamp, L.; Trosko, J.; Mansour, V. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Reedijk, J. New clues for platinum antitumor chemistry: Kinetically controlled metal binding to DNA. Proc. Natl. Acad. Sci. USA 2003, 100, 3611–3616. [Google Scholar] [CrossRef]
- Kostova, I. Platinum complexes as anticancer agents. Recent Pat. Anticancer Drug Discov. 2006, 1, 1–22. [Google Scholar] [CrossRef]
- Reedijk, J. Improved understanding in platinium antitumour chemistry. Chem. Commun. 1996, 7, 801–806. [Google Scholar] [CrossRef]
- Guo, Z.; Sadler, P.J. Medicinal inorganic chemistry. Adv. Inorg. Chem. 1999, 49, 183–306. [Google Scholar]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Casini, A.; Messori, L. Molecular mechanisms and proposed targets for selected anticancer gold compounds. Curr. Top. Med. Chem. 2011, 11, 2647–2660. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.G.; Keppler, B.K. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 2, 183–194. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Kapdi, A.R.; Fairlamb, I.J.S. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef] [PubMed]
- Navara-Ranninger, C.; Zamora, F.; Masaguer, J.R.; Perez, J.M.; Gonzalez, V.M.; Alonso, C. Palladium(II) compounds of putrescine and spermine. Synthesis, characterization, and DNA-binding and antitumor properties. J. Inorg. Biochem. 1993, 52, 37–49. [Google Scholar] [CrossRef]
- Karami, K.; Jamshidian, N.; Bagheri, A.; Hajiaghasi, A.; Momtazi-Borojeni, A.A.; Abdollahi, E.; Shahpiri, A.; Azizi, N.; Lipkowski, J. Novel fluorescence palladium-alkoxime complexes: Synthesis, characterization, DNA/BSA spectroscopic and docking studies, evaluation of cytotoxicity and DNA cleavage mechanism. J. Mol. Struct. 2020, 1206, 127595–127609. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Y.; Ge, J.; Lin, X.; Liu, D.; Wang, S.; Zhang, J.; Zhou, G.; Li, S. Synthesis, characterization, DNA/BSA interactions and in vitro cytotoxicity study of palladium(II) complexes of hispolon derivatives. J. Inorg. Biochem. 2020, 202, 110857–110869. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic Anticancer Compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.L.W.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Morsali, A.; Ramaxani, A.; Mahjoub, A.R. Ni(II), Pd(II), Cu(II) and Zn(II) complexes with N-(2-pyridyl)carbonylaniline (L), synthesis and X-ray crystal structures of [Cu(L)2(H2O)2](NO3)2, [Cu(L)2(H2O)2](ClO4)2 and [Zn(L)2(H2O)2](ClO4)2. J. Coord. Chem. 2003, 56, 1555–1566. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Duan, C.; Shao, Y.; Ding, J.; Miao, Z.; You, X.-Z.; Guo, Z. Structural evidence for the facile chelate-ring opening reactions of novel platinum(II)–pyridine carboxamide complexes. Dalton Trans. 2002, 591–597. [Google Scholar] [CrossRef]
- Dasa, D.; Muruganantham, A.; Kannan, S.; Kumar, M.; Sundararajan, M.; Sureshkumar, M.K. Coordination diversity in palladium(II)-picolinamide ligand complexes: Structural and quantum chemical studies. J. Coord. Chem. 2017, 70, 1548–1553. [Google Scholar] [CrossRef]
- Wilson, C.R.; Munro, O.Q. Unconventional hydrogen bonding and π-stacking in two substituted pyridine carboxamides. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2010, 66, o513–o616. [Google Scholar] [CrossRef]
- Dutta, S.; Pal, S.; Bhattacharya, P.K. Synthesis and characterisation of some Ru(II) complexes of 2-carbamoylpyridine derivatives. Polyhedron. 1999, 18, 2157–2162. [Google Scholar] [CrossRef]
- Shi, C.Y.; Gao, E.J.; Mab, S.; Wang, M.L.; Liu, Q.T. Synthesis, crystal structure, DNA-binding and cytotoxicity in vitro of novel cis-Pt(II) and trans-Pd(II) pyridine carboxamide complexes. Bioorg. Med. Chem. Lett. 2010, 20, 7250–7254. [Google Scholar] [CrossRef]
- CCD CrysAlis. CrysAlis Red; Xcalibur PX Software; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- SAINT, B. (v7.68A); Data Reduction Software. Bruker AXS Inc.: Madison, WI, USA, 2009.
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
| Atom | Length/Å | Atom | Angle/° |
|---|---|---|---|
| Pd1-N21 | 2.0361(19) | N21-Pd1-N2 | 180.0 |
| Pd1-N2 | 2.0361(19) | N2-Pd1-N1 | 80.35(8) |
| Pd1-N11 | 2.0399(19) | N21-Pd1-N11 | 80.35(8) |
| Pd1-N1 | 2.0400(19) | N2-Pd1-N11 | 99.65(8) |
| N21-Pd1-N1 | 99.65(8) | ||
| 11-X, 1-Y, 1-Z | N11-Pd1-N1 | 180.0 |
| Identification Code for C1 | cu_SS_PM_Br_Pd_Comp_0m |
|---|---|
| Empirical formula | C12H8BrN2OPd0.5 |
| Formula weight | 329.31 |
| Temperature (K) | 99.97 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a (Å) | 6.22590(10) |
| b/Å | 12.9253(3) |
| c (Å) | 13.6735(3) |
| α (°) | 90 |
| β (°) | 94.8170(10) |
| γ (°) | 90 |
| Volume (Å3) | 1096.44(4) |
| Z | 4 |
| ρcalc (g cm−3) | 1.995 |
| μ (mm−1) | 11.358 |
| F (000) | 640.0 |
| Crystal size (mm3) | 0.205 × 0.075 × 0.065 |
| Radiation source, λ (Å) | Cu(Kα), λ = 1.54178 |
| 2θ range for data collection (°) | 9.432 to 144.36 |
| Index ranges | −7 ≤ h ≤ 6, −15 ≤ k ≤ 15, −16 ≤ l ≤ 16 |
| Reflections collected | 14,012 |
| Independent reflections | 2097 [Rint = 0.0247, Rσ = 0.0160] |
| Data/restraints/parameters | 2097/0/151 |
| Goodness-of-fit on F2 | 1.125 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0213, wR2 = 0.0513 |
| Final R indexes (all data) | R1 = 0.0216, wR2 = 0.0515 |
| Largest diff. peak/hole (e Å−3) | 0.49/−0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mjwara, P.N.; Papo, T.R.; Sithebe, S. bis[N-(4-Bromophenyl)pyridine-2-carboxamidato]palladium. Molbank 2022, 2022, M1496. https://doi.org/10.3390/M1496
Mjwara PN, Papo TR, Sithebe S. bis[N-(4-Bromophenyl)pyridine-2-carboxamidato]palladium. Molbank. 2022; 2022(4):M1496. https://doi.org/10.3390/M1496
Chicago/Turabian StyleMjwara, Pinky N., Tshephiso R. Papo, and Siphamandla Sithebe. 2022. "bis[N-(4-Bromophenyl)pyridine-2-carboxamidato]palladium" Molbank 2022, no. 4: M1496. https://doi.org/10.3390/M1496
APA StyleMjwara, P. N., Papo, T. R., & Sithebe, S. (2022). bis[N-(4-Bromophenyl)pyridine-2-carboxamidato]palladium. Molbank, 2022(4), M1496. https://doi.org/10.3390/M1496