3-Morpholino-7-[N-methyl-N-(4′-carboxyphenyl)amino]phenothiazinium Chloride
Abstract
:1. Introduction
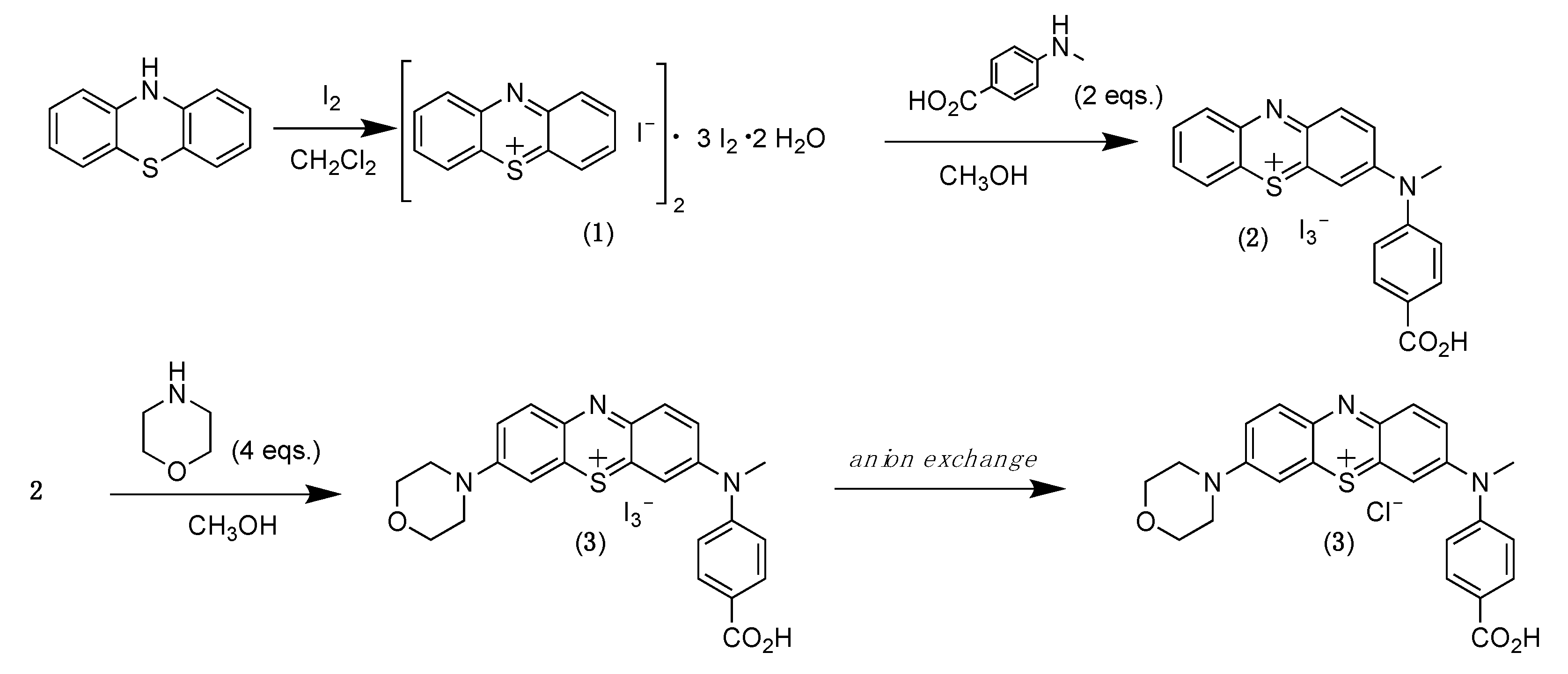
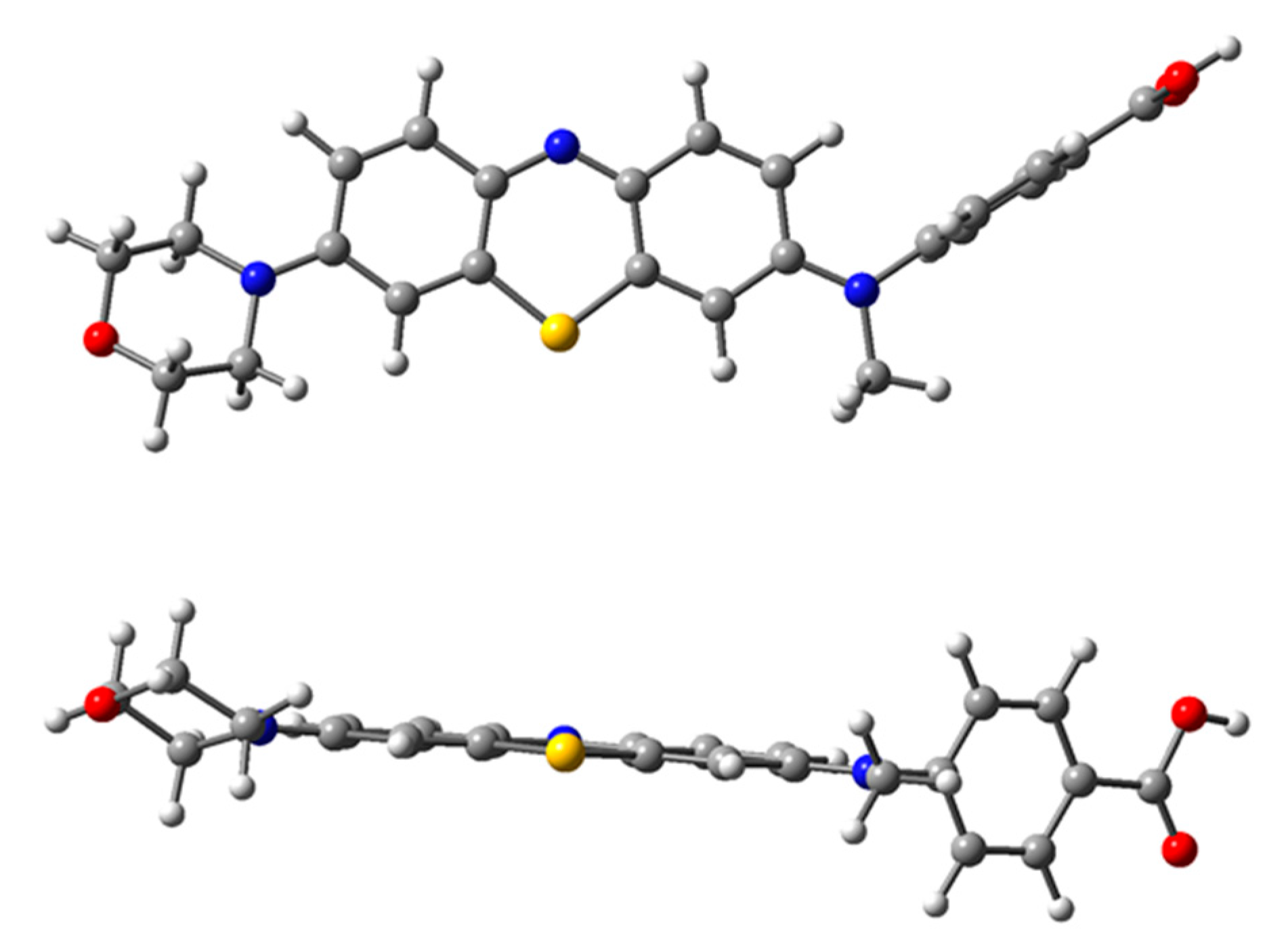
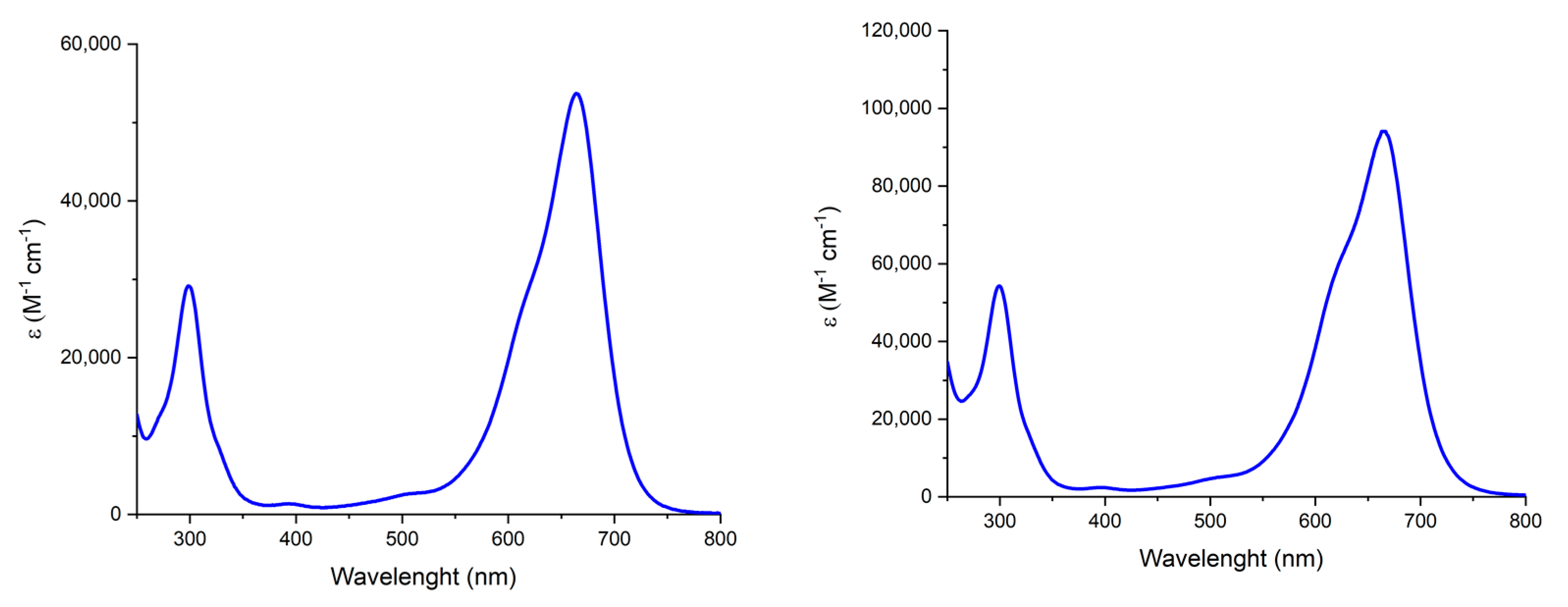
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitchell, S.C. Phenothiazine: The Parent Molecule. Curr. Drug Targets 2006, 61, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Al-Busaidi, I.J.; Haque, A.; Al Rasbi, N.K.; Khan, M.S. Phenothiazine-based derivatives for optoelectronic applications: A review. Synth. Met. 2019, 257, 116189. [Google Scholar] [CrossRef]
- Kamat, P.V.; Lichtln, N.N. Electron Transfer in the Quenching of Protonated Triplet Methylene Blue by Ground-State Molecules of the Dye. J. Phys. Chem. 1981, 85, 814–818. [Google Scholar] [CrossRef]
- Lal, C. Use of mixed dyes in a photogalvanic cell for solar energy conversion and storage: EDTA–thionine–azur-B system. J. Power Sources 2007, 164, 926–930. [Google Scholar] [CrossRef]
- Ye, J.; Baldwin, R.P. Catalytic reduction of myoglobin and hemoglobin at chemically modified electrodes containing methylene blue. Anal. Chem. 1988, 60, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Tuite, E.; Norden, B. Sequence-Specific Interactions of Methylene Blue with Polynucleotides and DNA: A Spectroscopic Study. J. Am. Chem. Soc. 1994, 116, 7548–7556. [Google Scholar] [CrossRef]
- Gorman, S.A.; Bell, A.L.; Griffiths, J.; Roberts, D.; Brown, S.B. The synthesis and properties of unsymmetrical 3,7-diaminophenothiazin-5-ium iodide salts: Potential photosensitisers for photodynamic therapy. Dyes Pigments 2006, 71, 153–160. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, S.; Ding, H.; Niu, F.; Chu, Y.; Wu, W.; Hu, Y.; Hu, K.; Hua, J. Pure organic quinacridone dyes as dual sensitizers in tandem photoelectrochemical cells for unassisted total water splitting. Chem. Commun. 2021, 57, 5634–5637. [Google Scholar] [CrossRef] [PubMed]
- Sabuzi, F.; Lentini, S.; Sforza, F.; Pezzola, S.; Fratelli, S.; Bortolini, O.; Floris, B.; Conte, V.; Galloni, P. KuQuinones Equilibria Assessment for Biomedical Applications. J. Org. Chem. 2017, 82, 10129–10138. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Phan, H.; Herng, T.S.; Hu, P.; Zeng, W.; Dong, S.-Q.; Das, S.; Shen, Y.; Ding, J.; Casanova, D.; et al. Higher Order π-Conjugated Polycyclic Hydrocarbons with Open-Shell Singlet Ground State: Nonazethrene versus Nonacene. J. Am. Chem. Soc. 2016, 138, 10323–10330. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, S.; Ding, J.; Wang, R.; Zhang, Y. Effect of π-conjugation on solid-state fluorescence in highly planar dyes bearing an intramolecular H-bond. Dye. Pigment. 2020, 182, 108665. [Google Scholar] [CrossRef]
- Tiravia, M.; Sabuzi, F.; Cirulli, M.; Pezzola, S.; Di Carmine, G.; Cicero, D.O.; Floris, B.; Conte, V.; Galloni, P. 3,7-Bis(N-methyl-N-phenylamino)phenothiazinium Salt: Improved Synthesis and Aggregation Behavior in Solution. Eur. J. Org. Chem. 2019, 2019, 3208–3216. [Google Scholar] [CrossRef]
- Stoean, B.; Gaina, L.; Cristea, C.; Silaghi-Dumitrescu, R.; Branzanic, A.M.V.; Focsan, M.; Fischer-Fodor, E.; Tigu, B.; Moldovan, C.; Cecan, A.D.; et al. New methylene blue analogues with N-piperidinyl-carbinol units: Synthesis, optical properties and in vitro internalization in human ovarian cancer cells. Dyes Pigments 2022, 205, 110460. [Google Scholar] [CrossRef]
- Kirla, H.; Henry, D.J. Synthesis and characterization of novel silane derivatives of phenothiazinium photosensitisers. Dye. Pigment. 2022, 199, 110087. [Google Scholar] [CrossRef]
- Kannaiyan, S.; Easwaramoorthy; Kannan, K.; Andal, V. Green synthesis of Phenothiazinium Schiff base and its nano silver complex using egg white as a catalyst under solvent free condition. Mater. Today-Proc. 2022, 55, 267–273. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.A.; Marasi, M.; Gobbato, T.; Valentini, F.; Sabuzi, F.; Gagliardi, V.; Bonetto, A.; Marcomini, A.; Berardi, S.; Conte, V.; et al. Photoanodes for water oxidation with visible light based on a pentacyclic quinoid organic dye enabling proton-coupled electron transfer. Chem. Commun. 2020, 56, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.; Sabuzi, F.; Di Carlo, A.; Conte, V.; Dini, D.; Galloni, P. KuQuinones as sensitizers of NiO based p-type dye sensitized solar cells. New J. Chem. 2017, 41, 2769–2779. [Google Scholar] [CrossRef]
- Strekowski, L.; Hou, D.-F.; Wydra, R.L.; Schinazi, R.F. A synthetic route to 3-(dialkylamino)phenothiazin-5-ium salts and 3,7-disubstituted derivatives containing two different amino groups. J. Heterocycl. Chem. 1993, 30, 1693–1695. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiravia, M.; Sabuzi, F.; Valentini, F.; Conte, V.; Galloni, P. 3-Morpholino-7-[N-methyl-N-(4′-carboxyphenyl)amino]phenothiazinium Chloride. Molbank 2022, 2022, M1493. https://doi.org/10.3390/M1493
Tiravia M, Sabuzi F, Valentini F, Conte V, Galloni P. 3-Morpholino-7-[N-methyl-N-(4′-carboxyphenyl)amino]phenothiazinium Chloride. Molbank. 2022; 2022(4):M1493. https://doi.org/10.3390/M1493
Chicago/Turabian StyleTiravia, Martina, Federica Sabuzi, Francesca Valentini, Valeria Conte, and Pierluca Galloni. 2022. "3-Morpholino-7-[N-methyl-N-(4′-carboxyphenyl)amino]phenothiazinium Chloride" Molbank 2022, no. 4: M1493. https://doi.org/10.3390/M1493
APA StyleTiravia, M., Sabuzi, F., Valentini, F., Conte, V., & Galloni, P. (2022). 3-Morpholino-7-[N-methyl-N-(4′-carboxyphenyl)amino]phenothiazinium Chloride. Molbank, 2022(4), M1493. https://doi.org/10.3390/M1493






