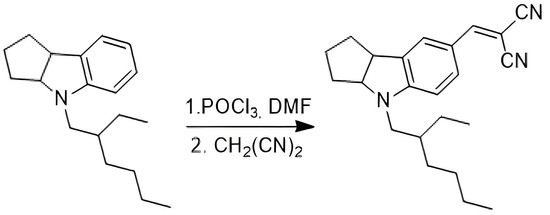
2-((4-(2-Ethylhexyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indol-7-yl)methylene)malononitrile
Abstract
:1. Introduction
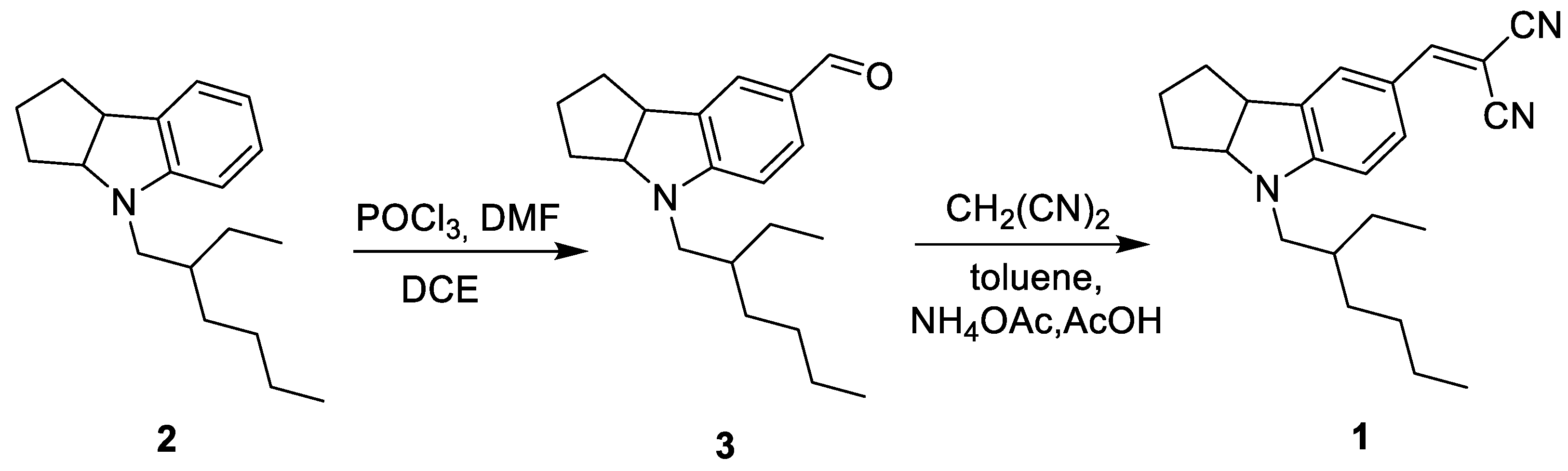
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and Opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.-C.; Wong, W.-Y. Recent progress of electronic materials based on 2,1,3-benzothiadiazole and its derivatives: Synthesis and their application in organic light-emitting diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- van der Staaij, F.M.; van Keulen, I.M.; von Hauff, E. Organic Photovoltaics: Where Are We Headed? Sol. RRL 2021, 5, 2100167. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Okura, T.; Okuda, Y.; Matsui, J.; Masuhara, A.; Yoshida, T.; White, M.S.; Yumusak, C.; Stadler, P.; Scharber, M.; et al. Single-Component Organic Solar Cells Based on Intramolecular Charge Transfer Photoabsorption. Materials 2021, 14, 1200. [Google Scholar] [CrossRef] [PubMed]
- Terenti, N.; Giurgi, G.-I.; Crişan, A.P.; Anghel, C.; Bogdan, A.; Pop, A.; Stroia, I.; Terec, A.; Szolga, L.; Grosu, I.; et al. Structure–properties of small donor–acceptor molecules for homojunction single-material organic solar cells. J. Mater. Chem. C 2022, 10, 5716–5726. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Kotowicz, S.; Szafraniec-Gorol, G.; Zimosz, S.; Schab-Balcerzak, E.; Siwy, M.; Grzelak, J.; Maćkowski, S. “Small in size but mighty in force”—The first principle study of the impact of A/D units in A/D-phenyl-π-phenothiazine-π-dicyanovinyl systems on photophysical and optoelectronic properties. Dye. Pigment. 2021, 189, 109248. [Google Scholar] [CrossRef]
- Matsui, M.; Fujita, T.; Kubota, Y.; Funabiki, K.; Jin, J.; Yoshida, T.; Miura, H. Substituent effects in a double rhodanine indoline dye on performance of zinc oxide dye-sensitized solar cell. Dye. Pigment. 2010, 86, 143–148. [Google Scholar] [CrossRef]
- Matsui, M.; Shiota, T.; Kubota, Y.; Funabiki, K.; Jin, J.; Yoshida, T.; Higashijima, S.; Miura, H. N-(2-Alkoxyphenyl)-substituted double rhodanine indoline dyes for zinc oxide dye-sensitized solar cell. Tetrahedron 2012, 68, 4286–4291. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, R.A. An investigation on single crystal growth, structural, thermal and optical properties of a series of organic D–π–A push–pull materials. RSC Adv. 2015, 5, 38591–38600. [Google Scholar] [CrossRef]
- Tanaka, T.; Watanabe, K.; Nomoto, T.; Okano, M.; Shintou, T.; Miyazaki, T.; Nishimura, Y.; Shimada, Y. Evaluation Probe for Central Nervous System Permeability, Evaluation Method for Central Nervous System Permeability, and Screening Method Using an Evaluation Probe for Central Nervous System Permeability. U.S. Patent 10,227,337, 12 March 2019. [Google Scholar]
- Tanaka, E.; Mikhailov, M.S.; Gudim, N.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. Structural features of indoline donors in D–A-π-A type organic sensitizers for dye-sensitized solar cells. Mol. Syst. Des. Engl. 2021, 6, 730–738. [Google Scholar] [CrossRef]
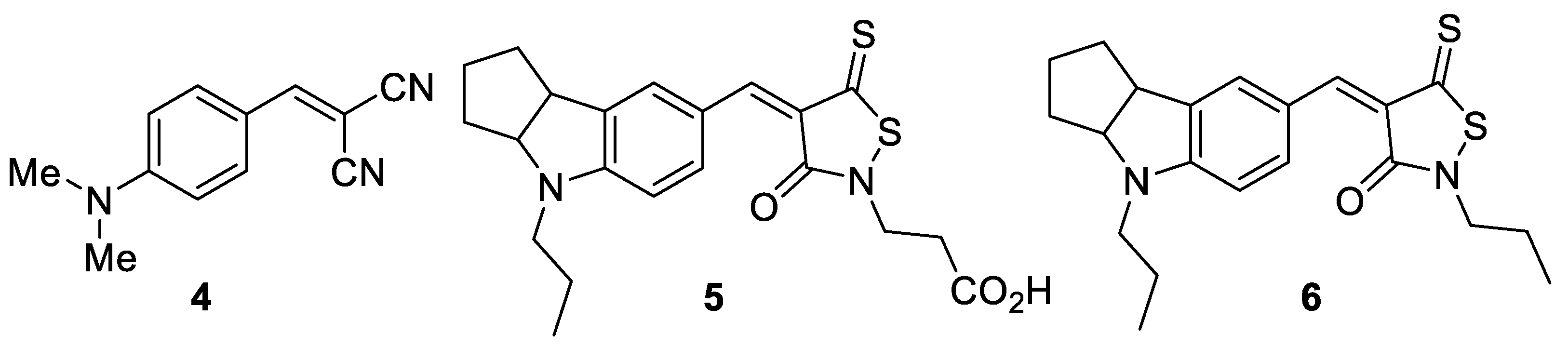
| Compound | λabs nm | εmax mol × 1−1 × cm−1 | λem nm | Stokes Shift ∆ν cm−1 |
|---|---|---|---|---|
| 1 | 462 | 97,028 | 510 | 2038 |
| 4 | 447 | 81,008 | 557 | 4418 |
| 5 | 519 | – | 592 | 2376 |
| 6 | 517 | – | 590 | 2393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudim, N.S.; Knyazeva, E.A.; Trainov, K.P.; Rakitin, O.A. 2-((4-(2-Ethylhexyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indol-7-yl)methylene)malononitrile. Molbank 2022, 2022, M1490. https://doi.org/10.3390/M1490
Gudim NS, Knyazeva EA, Trainov KP, Rakitin OA. 2-((4-(2-Ethylhexyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indol-7-yl)methylene)malononitrile. Molbank. 2022; 2022(4):M1490. https://doi.org/10.3390/M1490
Chicago/Turabian StyleGudim, Nikita S., Ekaterina A. Knyazeva, Konstantin P. Trainov, and Oleg A. Rakitin. 2022. "2-((4-(2-Ethylhexyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indol-7-yl)methylene)malononitrile" Molbank 2022, no. 4: M1490. https://doi.org/10.3390/M1490
APA StyleGudim, N. S., Knyazeva, E. A., Trainov, K. P., & Rakitin, O. A. (2022). 2-((4-(2-Ethylhexyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indol-7-yl)methylene)malononitrile. Molbank, 2022(4), M1490. https://doi.org/10.3390/M1490