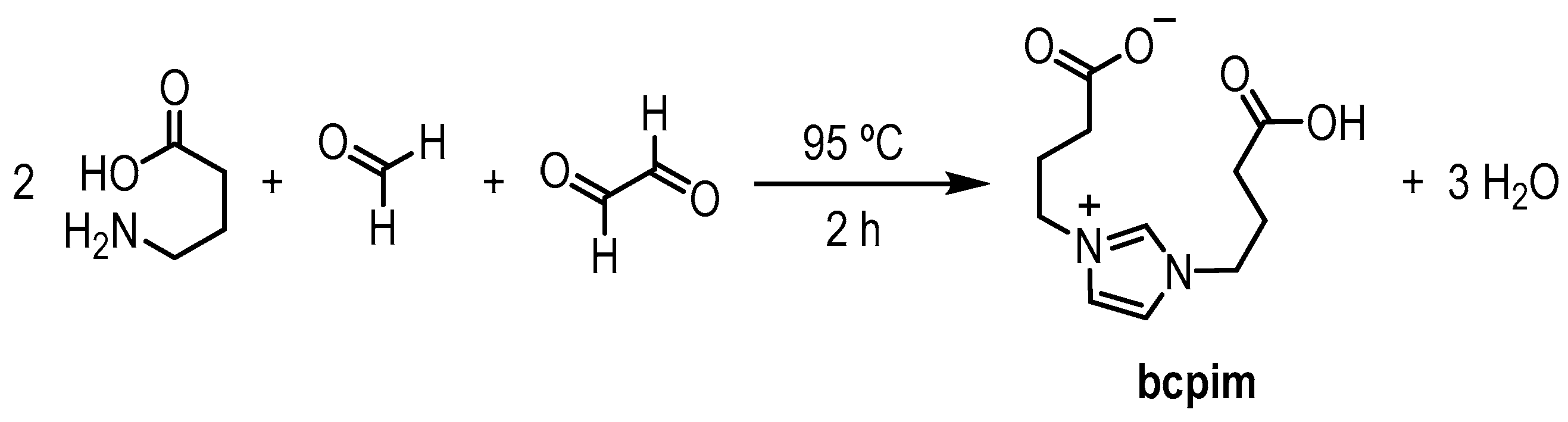
1,3-Bis(3-carboxypropyl)-1H-imidazole
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keaveney, S.T.; White, B.P.; Haines, R.S.; Harper, J.B. The effects of an ionic liquid on unimolecular substitution processes: The importance of the extent of transition state solvation. Org. Biomol. Chem. 2016, 14, 2572–2580. [Google Scholar] [CrossRef]
- Liu, Q.; Janssen, M.H.A.; van Rantwijk, F.; Sheldon, R.A. Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem. 2005, 7, 39–42. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Cavell, K.J. Donor-Functionalized Heterocyclic Carbene Complexes of Palladium(II): Efficient Catalysts for C−C Coupling Reactions. Organometallics 2000, 19, 741–748. [Google Scholar] [CrossRef]
- Bolaño, T.; Esteruelas, M.A.; Gay, M.P.; Oñate, E.; Pastor, I.M.; Yus, M. An Acyl-NHC Osmium Cooperative System: Coordination of Small Molecules and Heterolytic B–H and O–H Bond Activation. Organometallics 2015, 34, 3902–3908. [Google Scholar] [CrossRef]
- Allegue, A.; Albert-Soriano, M.; Pastor, I.M. A comparative study of hydroxyl- and carboxylate-functionalized imidazolium and benzimidazolium salts as precursors for N-heterocyclic carbene ligands. Appl. Organomet. Chem. 2015, 29, 624–632. [Google Scholar] [CrossRef]
- Trillo, P.; Pastor, I.M. Iron-Based Imidazolium Salts as Versatile Catalysts for the Synthesis of Quinolines and 2- and 4-Allylanilines by Allylic Substitution of Alcohols. Adv. Synth. Catal. 2016, 358, 2929–2939. [Google Scholar] [CrossRef]
- Martos, M.; Pastor, I.M. Imidazolium-urea low transition temperature mixtures for the UHP-promoted oxidation of boron compounds. J. Mol. Liq. 2022, 347, 118349. [Google Scholar] [CrossRef]
- Martos, M.; Pastor, I.M. Iron-Based Imidazolium Salt as Dual Lewis Acid and Redox Catalyst for the Aerobic Synthesis of Quinazolines. Eur. J. Org. Chem. 2022, 2022, e202200839. [Google Scholar] [CrossRef]
- Gisbert, P.; Trillo, P.; Pastor, I.M. Comparative Study of Catalytic Systems Formed by Palladium and Acyl-Substituted Imidazolium Salts. ChemistrySelect 2018, 3, 887–893. [Google Scholar] [CrossRef]
- Dewilde, S.; Dehaen, W.; Binnemans, K. Ionic liquids as solvents for PPTA oligomers. Green Chem. 2015, 18, 1639–1652. [Google Scholar] [CrossRef]
- Arduengo, A.J., III. Preparation of 1,3-Disubstituted Imidazolium Salts 1991. U.S. Patent 5182405-A, 26 January 1993. [Google Scholar]
- Rossi, R.; Angelici, G.; Casotti, G.; Manzini, C.; Lessi, M. Catalytic Synthesis of 1,2,4,5-Tetrasubstituted 1 H-Imidazole Derivatives: State of the Art. Adv. Synth. Catal. 2019, 361, 2737–2803. [Google Scholar] [CrossRef]
- Kühl, O.; Palm, G. Imidazolium salts from amino acids—A new route to chiral zwitterionic carbene precursors? Tetrahedron Asymmetry 2010, 21, 393–397. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Hernández-Martínez, L.; Pastor, I.M. 1,3-Bis(carboxymethyl)imidazolium Chloride as a Metal-Free and Recyclable Catalyst for the Synthesis of N-Allylanilines by Allylic Substitution of Alcohols. ACS Sustain. Chem. Eng. 2018, 6, 14063–14070. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Pastor, I.M. Metal-Organic Framework Based on Copper and Carboxylate-Imidazole as Robust and Effective Catalyst in the Oxidative Amidation of Carboxylic Acids and Formamides. Eur. J. Org. Chem. 2016, 2016, 5180–5188. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Trillo, P.; Soler, T.; Pastor, I.M. Versatile Barium and Calcium Imidazolium-Dicarboxylate Heterogeneous Catalysts in Quinoline Synthesis. Eur. J. Org. Chem. 2017, 2017, 6375–6381. [Google Scholar] [CrossRef]
- Gisbert, P.; Albert-Soriano, M.; Pastor, I.M. Effective and Sustainable Access to Quinolines and Acridines: A Heterogeneous Imidazolium Salt Mediates C-C and C-N Bond Formation. Eur. J. Org. Chem. 2019, 2019, 4928–4940. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Pastor, I.M. Anion-Dependent Imidazolium-Based Catalysts for Allylation of Aniline with Tunable Regioselectivity. Adv. Synth. Catal. 2020, 362, 2494–2502. [Google Scholar] [CrossRef]
- Gisbert, P.; Pastor, I.M. Efficient Thiophene Synthesis Mediated by 1,3-Bis(carboxymethyl)imidazolium Chloride: C-C and C-S Bond Formation. Eur. J. Org. Chem. 2020, 2020, 4319–4325. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Brønsted Acidic Ionic Liquids and Their Zwitterions: Synthesis, Characterization and pKa Determination. Chem.—Eur. J. 2004, 10, 4886–4893. [Google Scholar] [CrossRef]
- Dai, Q.; Ma, J.; Ma, S.; Wang, S.; Li, L.; Zhu, X.; Qiao, X. Cationic Ionic Liquids Organic Ligands Based Metal–Organic Frameworks for Fabrication of Core–Shell Microspheres for Hydrophilic Interaction Liquid Chromatography. ACS Appl. Mater. Interfaces 2016, 8, 21632–21639. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Andraos, J. Reaction Green Metrics—Problems, Exercises, and Solutions; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar]
| Parameter | Value | ||
|---|---|---|---|
| This Work | Ref. [20] | ||
| E-factor (Total) | 8.41 | 93.34 | |
| E-factor (kernel) | 0.43 | 1.71 | |
| E-factor (excess) | 0.00 | 0.09 | |
| E-factor (catalyst) | 0.00 | 0.00 | |
| E-factor (solvent) | 0.67 | 13.33 | |
| E-factor (work-up) | 0.00 | 0.00 | |
| E-factor (purification) | 7.31 | 78.21 | |
| EcoScale | 80 | 58 | |
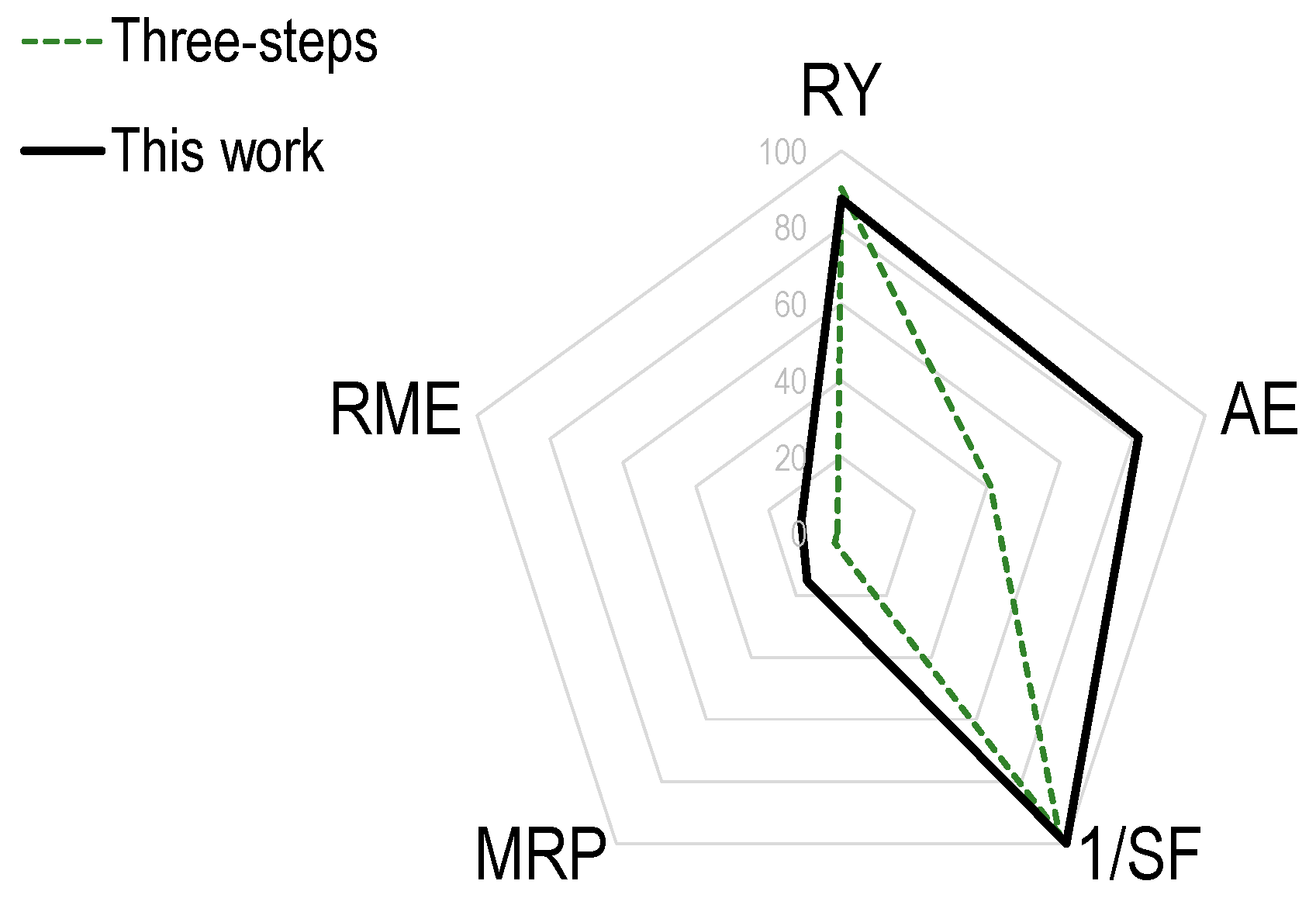
| Parameter | Value (%) 1 | |
|---|---|---|
| This Work | Ref. [20] | |
| Reaction yield (RY) | (87%) | (90%) |
| Atom economy (AE) | 0.816 (82%) | 0.409 (41%) |
| Stoichiometric factor (SF) | 0.999 | 1.032 |
| 1/SF | 1.00 (100%) | 0.969 (97%) |
| Reaction mass efficiency (RME) | 0.108 (11%) | 0.011 (1%) |
| Materials recovery parameter (MRP) | 0.152 (15%) | 0.030 (3%) |
| Vector magnitude ratio (VMR) 2 | 0.702 | 0.619 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos, M.; Albert-Soriano, M.; Pastor, I.M. 1,3-Bis(3-carboxypropyl)-1H-imidazole. Molbank 2022, 2022, M1480. https://doi.org/10.3390/M1480
Martos M, Albert-Soriano M, Pastor IM. 1,3-Bis(3-carboxypropyl)-1H-imidazole. Molbank. 2022; 2022(4):M1480. https://doi.org/10.3390/M1480
Chicago/Turabian StyleMartos, Mario, María Albert-Soriano, and Isidro M. Pastor. 2022. "1,3-Bis(3-carboxypropyl)-1H-imidazole" Molbank 2022, no. 4: M1480. https://doi.org/10.3390/M1480
APA StyleMartos, M., Albert-Soriano, M., & Pastor, I. M. (2022). 1,3-Bis(3-carboxypropyl)-1H-imidazole. Molbank, 2022(4), M1480. https://doi.org/10.3390/M1480







