4a,7a-Dihydroxy-1-(2-hydroxyethyl)-5-methyl-2′,3′,4a,5′,6′,7a-hexahydrospiro[cyclopenta[b]pyridine-4,4′-pyran]-2,7(1H,3H)-dione
Abstract
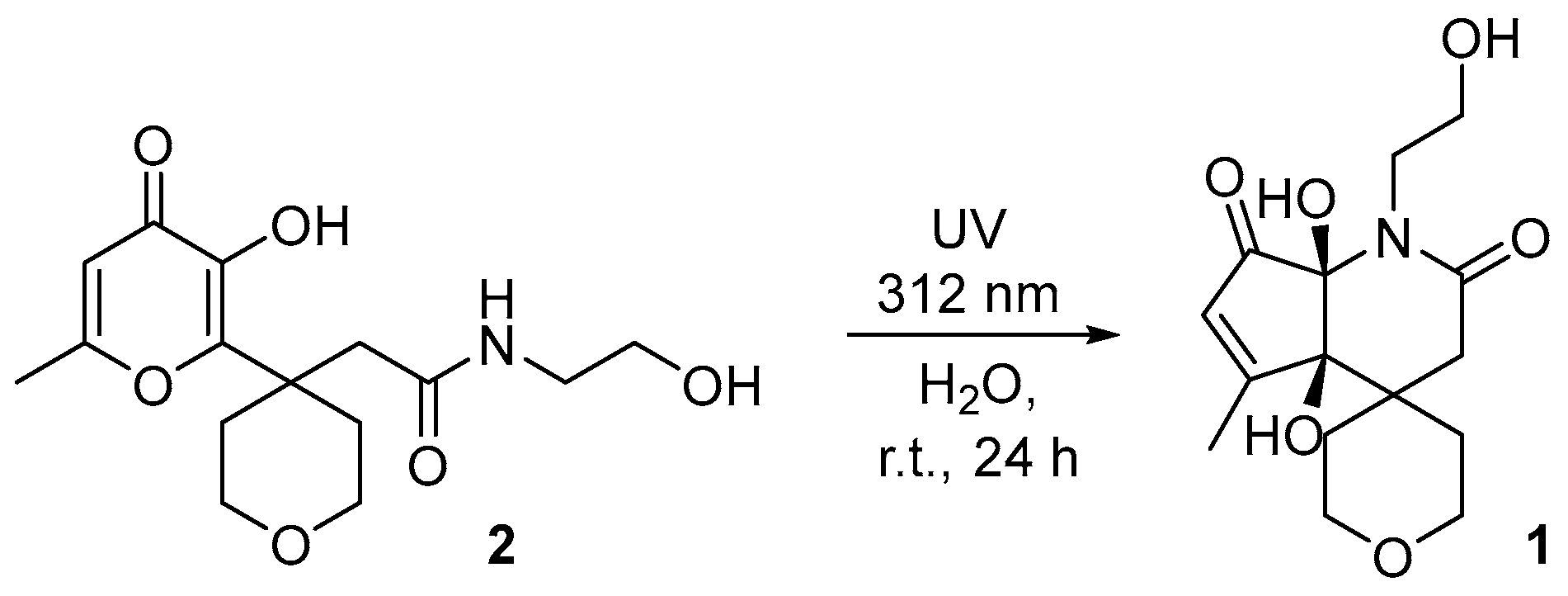
1. Introduction
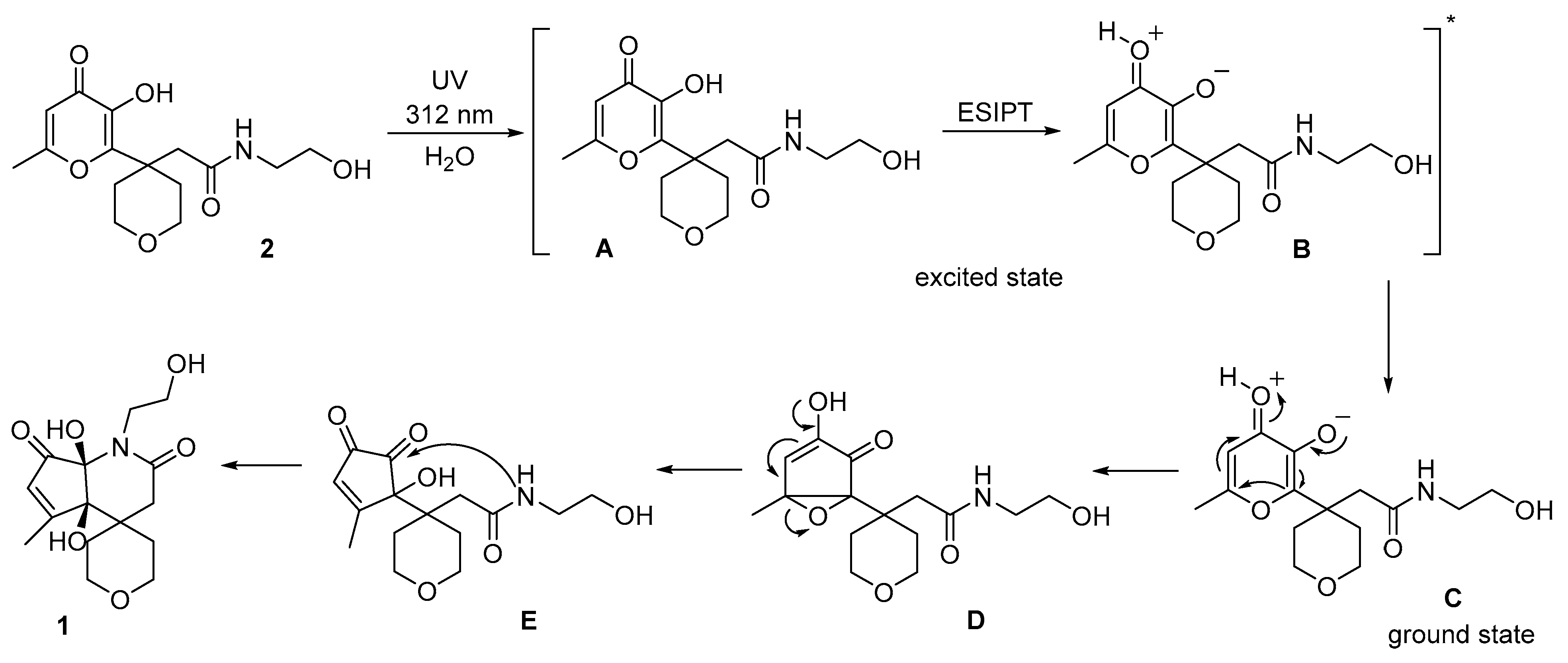
2. Results
3. Materials and Methods
4. Conclusions
Experimental Procedure for the Synthesis of 4a,7a-Dihydroxy-1-(2-hydroxyethyl)-5-methyl-2′,3′,4a,5′,6′,7a-hexahydrospiro[cyclopenta[b]pyridine-4,4′-pyran]-2,7(1H,3H)-dione 1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kärkäs, M.D.; Porco, J.A., Jr.; Stephenson, C.R.J. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar] [CrossRef] [PubMed]
- Bach, T.; Hehn, J.P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem. Int. Ed. 2011, 50, 1000–1045. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y.-J.; Liang, X.-W. Total synthesis of natural products using photocycloaddition reactions of arenes. Org. Biomol. Chem. 2020, 18, 5558–5566. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Bera, N.; Ghosh, S. [2+2] Photochemical Cycloaddition in Organic Synthesis: [2+2] Photochemical Cycloaddition in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 1310–1326. [Google Scholar] [CrossRef]
- Kaur, N. Photochemical Reactions for the Synthesis of Six-Membered O-Heterocycles. Curr. Org. Synth. 2018, 15, 298–320. [Google Scholar] [CrossRef]
- Di Filippo, M.; Bracken, C.; Baumann, M. Continuous Flow Photochemistry for the Preparation of Bioactive Molecules. Molecules 2020, 25, 356. [Google Scholar] [CrossRef]
- Albini, A.; Fagnoni, M. Green chemistry and photochemistry were born at the same time. Green Chem. 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Protti, S.; Dondi, D.; Fagnoni, M.; Albini, A. Assessing photochemistry as a green synthetic method. Carbon–carbon bond forming reactions. Green Chem. 2009, 11, 239–249. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Milyutin, C.V.; Komogortsev, A.N.; Lichitsky, B.V.; Melekhina, V.G. A study of the photochemical behavior of terarylenes containing allomaltol and pyrazole fragments. Beilstein J. Org. Chem. 2022, 18, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Milyutin, C.V.; Komogortsev, A.N.; Lichitsky, B.V.; Melekhina, V.G.; Minyaev, M.E. Construction of Spiro-γ-butyrolactone Core via Cascade Photochemical Reaction of 3-Hydroxypyran-4-one Derivatives. Org. Lett. 2021, 23, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Komogortsev, A.N.; Lichitsky, B.V.; Melekhina, V.G.; Nasyrova, D.I.; Milyutin, C.V. Photoinduced 6π-Electrocyclization of a 1,3,5-Hexatriene System Containing an Allomaltol Fragment. J. Org. Chem. 2021, 86, 15345–15356. [Google Scholar] [CrossRef] [PubMed]
- Melekhina, V.G.; Mityanov, V.S.; Lichitsky, B.V.; Komogortsev, A.N.; Lyssenko, K.A.; Krayushkin, M.M. Synthesis of Benzocarbazole Derivatives by Photocyclization. Eur. J. Org. Chem. 2019, 2019, 1335–1340. [Google Scholar] [CrossRef]
- Melekhina, V.G.; Mityanov, V.S.; Lichitsky, B.V.; Komogortsev, A.N.; Fakhrutdinov, A.N.; Daeva, E.D.; Krayushkin, M.M. Ultraviolet irradiation of terarylenes: A facile, efficient, and environmentally friendly method for the synthesis of fused polycyclic products. Tetrahedron Lett. 2019, 60, 1745–1747. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Karibov, T.T.; Melekhina, V.G.; Komogortsev, A.N.; Fakhrutdinov, A.N.; Minyaev, M.E.; Krayushkin, M.M. General approach to substituted naphtho [1, 2-b]benzofurans via photochemical 6π-electocyclization of benzofuranyl containing cinnamonitriles. Tetrahedron 2021, 90, 132207. [Google Scholar] [CrossRef]
- Karibov, T.T.; Lichitsky, B.V.; Melekhina, V.G.; Komogortsev, A.N. The First Example of Photogeneration of a Pyrrole Molecule on the Basis of 6π-Electrocyclization of 2-Arylbenzofurans Containing a Pyrazole Fragment. Polycycl. Aromat. Compd. 2022, 1–21. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Milyutin, C.V.; Melekhina, V.G.; Fakhrutdinov, A.N.; Komogortsev, A.N.; Krayushkin, M.M. Photochemical synthesis of novel naphto [1,2-b]benzofuran derivatives from 2,3-disubstituted benzofurans. Chem. Heterocycl. Compd. 2021, 57, 13–19. [Google Scholar] [CrossRef]
- Milyutin, C.V.; Galimova, R.D.; Komogortsev, A.N.; Lichitskii, B.V.; Melekhina, V.G.; Migulin, V.A.; Fakhrutdinov, A.N.; Minyaev, M.E. Photoinduced assembly of the 3,4,4a,7a-tetrahydro-1H-cyclopenta[b]pyridine-2,7-dione core on the basis of allomaltol derivatives. Org. Biomol. Chem. 2021, 19, 9975–9985. [Google Scholar] [CrossRef]
- Komogortsev, A.N.; Milyutin, C.V.; Lichitsky, B.V.; Melekhina, V.G. Photoinduced 6π-electicyclization of 1,3,5-hexatriene system containing allomaltol fragment: A convenient approach to polycondensed pyrrole derivatives. Tetrahedron 2022, 114, 132780. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.C.; Antonov, L. Excited-State Intramolecular Proton Transfer: A Short Introductory Review. Molecules 2021, 26, 1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Yang, D. Theoretical insights into excited-state hydrogen bonding effects and intramolecular proton transfer (ESIPT) mechanism for BTS system. Sci. Rep. 2020, 10, 5119. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komogortsev, A.N.; Lichitsky, B.V.; Melekhina, V.G. 4a,7a-Dihydroxy-1-(2-hydroxyethyl)-5-methyl-2′,3′,4a,5′,6′,7a-hexahydrospiro[cyclopenta[b]pyridine-4,4′-pyran]-2,7(1H,3H)-dione. Molbank 2022, 2022, M1481. https://doi.org/10.3390/M1481
Komogortsev AN, Lichitsky BV, Melekhina VG. 4a,7a-Dihydroxy-1-(2-hydroxyethyl)-5-methyl-2′,3′,4a,5′,6′,7a-hexahydrospiro[cyclopenta[b]pyridine-4,4′-pyran]-2,7(1H,3H)-dione. Molbank. 2022; 2022(4):M1481. https://doi.org/10.3390/M1481
Chicago/Turabian StyleKomogortsev, Andrey N., Boris V. Lichitsky, and Valeriya G. Melekhina. 2022. "4a,7a-Dihydroxy-1-(2-hydroxyethyl)-5-methyl-2′,3′,4a,5′,6′,7a-hexahydrospiro[cyclopenta[b]pyridine-4,4′-pyran]-2,7(1H,3H)-dione" Molbank 2022, no. 4: M1481. https://doi.org/10.3390/M1481
APA StyleKomogortsev, A. N., Lichitsky, B. V., & Melekhina, V. G. (2022). 4a,7a-Dihydroxy-1-(2-hydroxyethyl)-5-methyl-2′,3′,4a,5′,6′,7a-hexahydrospiro[cyclopenta[b]pyridine-4,4′-pyran]-2,7(1H,3H)-dione. Molbank, 2022(4), M1481. https://doi.org/10.3390/M1481





