(Z)-2-(1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethylidene)-N-phenylhydrazine-1-carbothioamide
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of 3
2.2. NMR Spectroscopic Analysis
2.3. X-ray Crystal Structure Description
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Data Collection and Structure Refinement Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogolino, D.; Gatti, A.; Carcelli, M.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, F.; Buschini, A.; Montalbano, S.; Feretti, D.; et al. Thiosemicarbazone scaffold for the design of antifungal and antiaflatoxigenic agents: Evaluation of ligands and related copper complexes. Sci. Rep. 2017, 7, 11214. [Google Scholar] [CrossRef]
- Guo, Z.L.; Richardson, D.R.; Kalinowski, D.S.; Kovacevic, Z.; Tan-Un, K.C.; Chan, G.C.-F. The novel thiosemicarbazone, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), inhibits neuroblastoma growth in vitro and in vivo via multiple mechanisms. J. Hematol. Oncol. 2016, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.A.; Bajaj, K.; Vishnosky, N.S.; Peterson, M.A.; Mashuta, M.S.; Buchanan, R.M.; Bates, P.J.; Grapperhaus, C.A. Synthesis, characterization, and biological activity of hybrid thiosemicarbazone–alkylthiocarbamate metal complexes. Inorg. Chem. 2020, 59, 4924–4935. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Jawaria, R.; Khan, M.U.; Braga, A.A.C.; Shafiq, Z.; Imran, M.; Zafar, H.M.A.; Irfan, A. An efficient synthesis, spectroscopic characterization, and optical nonlinearity response of novel salicylaldehyde thiosemicarbazone derivatives. ACS Omega 2021, 6, 16058–16065. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.Z.; Rizzk, Y.W.; El Deen, I.M.; Mourad, A.A.E.; El Behery, M. Design, synthesis, cytotoxic screening and molecular docking studies of novel hybrid yhiosemicarbazone derivatives as anticancer agents. Chem. Biodivers. 2021, 18, e2100580. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; García-Tasende, M.S.; Sordo, J. Main group metal complexes of semicarbazones and thiosemicarbazones. A structural review. Coordination Chem. Rev. 2000, 209, 197–261. [Google Scholar] [CrossRef]
- West, D.X.; Padhye, S.B.; Sonawane, P.B. Structural and physical correlations in the biological properties of transition metal heterocyclic thiosemicarbazone and S-alkyldithiocarbazate complexes. In Complex Chemistry; Springer: Berlin/Heidelberg, Germany, 1991; Volume 76. [Google Scholar] [CrossRef]
- Pelosi, G. Thiosemicarbazone metal complexes: From structure to activity. Open Crystallogr. J. 2010, 3, 16–28. [Google Scholar] [CrossRef]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal activity of thiosemicarbazones, bis(thiosemicarbazones), and their metal complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; Mayer, P. Copper complexes of new thiosemicarbazone ligands: Synthesis, structural studies and antimicrobial activity. Inorg. Chem. Commun. 2018, 94, 127–132. [Google Scholar] [CrossRef]
- El-Asmy, A.A.; Al-Hazmi, G.A.A. Synthesis and spectral feature of benzophenone-substituted thiosemicarbazones and their Ni(II) and Cu(II) complexes. Spectrochim. Acta A 2009, 71, 1885–1890. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, M.I.; Adnan, M.; et al. Therapeutic outcomes of isatin and its derivatives against multiple diseases: Recent developments in drug discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Yousef, T.A.; Abu El-Reash, G.M.; El-Gammal, O.A.; Bedier, R.A. Co(II), Cu(II), Cd(II), Fe(III) and U(VI) complexes containing a NSNO donor ligand: Synthesis, characterization, optical band gap, in vitro antimicrobial and DNA cleavage studies. J. Mol. Struct. 2012, 1029, 149–160. [Google Scholar] [CrossRef]
- Jawaria, R.; Hussain, M.; Khalid, M.; Khan, M.U.; Tahir, M.N.; Naseer, M.M.; Braga, A.A.C.; Shafiq, Z. Synthesis, crystal structure analysis, spectral characterization and nonlinear optical exploration of potent thiosemicarbazones based compounds: A DFT refine experimental study. Inorg. Chim. Acta 2019, 486, 162–171. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Li, Z.; Zhang, M.; Song, M. Voltammetric metal cation sensors based on ferrocenylthiosemicarbazone. Inorg. Chem. Commun. 2007, 10, 1485–1488. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Chira, N.A.; Pandele, A.M.; Hanganu, A.; AntonIvanova, A.; Tecuceanu, V.; Bugean, I.G.; Buica, G.-O. Electrodes modified with clickable thiosemicarbazone ligands for sensitive voltammetric detection of Hg(II) ions. Sens. Actuators B Chem. 2020, 313, 128030. [Google Scholar] [CrossRef]
- Ying, S.-M. Synthesis, crystal structure and nonlinear optical property of a zinc(II) complex base on the reduced Schiff-base ligand. Inorg. Chem. Commun. 2012, 22, 82–84. [Google Scholar] [CrossRef]
- Fernández-Luna, V.G.; Mallinson, D.; Alexiou, P.; Khadra, I.; Mullen, A.B.; Pelecanou, M.; Sagnou, M.; Lamprou, D.A. Isatin thiosemicarbazones promote honeycomb structure formation in spin-coated polymer films: Concentration effect and release studies. RSC Adv. 2017, 7, 12945–12952. [Google Scholar] [CrossRef]
- Marzi, M.; Farjam, M.; Kazeminejad, Z.; Shiroudi, A.; Kouhpayeh, A.; Zarenezhad, E. A recent overview of 1,2,3-triazole-containing hybrids as novel antifungal agents: Focusing on synthesis, mechanism of action, and structure-activity relationship (SAR). J. Chem. 2022, 2022, 7884316. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Alotaibi, M.H.; El-Hiti, G.A. Synthesis of new symmetrical N,N′-diacylhydrazines and 2-(1,2,3-triazol-4-yl)-1,3,4-oxadiazoles. Lett. Org. Chem. 2017, 14, 591–596. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Khidre, R.E.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of novel heterocycles using 1,2,3-triazole-4-carbohydrazides as precursors. J. Heterocycl. Chem. 2020, 57, 1055–1062. [Google Scholar] [CrossRef]
- Jadhav, R.P.; Raundal, U.N.; Patil, A.A.; Bobade, V.D. Synthesis and biological evaluation of a series of 1,4-disubstituted 1,2,3-triazole derivatives as possible antimicrobial agents. J. Saudi Chem. Soc. 2017, 21, 152–159. [Google Scholar] [CrossRef]
- Krishna, P.M.; Ramachary, D.B.; Peesapati, S. Azide–acetonitrile “click” reaction triggered by Cs2CO3: The atom-economic, high-yielding synthesis of 5-amino-1,2,3-triazoles. RSC Adv. 2015, 5, 62062–62066. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, N.B. Synthesis of 1H-1,2,3-triazole derivatives by the cyclization of aryl azides with 2-benzothiazolylacetonone, 1,3-benzo-thiazol-2-ylacetonitrile, and (4-aryl-1,3-thiazol-2-yl)acetonitriles. Chem. Heterocycl. Compd. 2009, 45, 483–488. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Liu, G.; Xu, H.; Song, L.; Chen, J.; Li, J.; Zhang, Z. Transition-metal-free synthesis of 5-amino-1,2,3-triazoles via nucleophilic addition/cyclization of carbodiimides with diazo compounds. Org. Chem. Front. 2021, 8, 599–604. [Google Scholar] [CrossRef]
- Opsomer, T.; Thomas, J.; Dehaen, W. Chemoselectivity in the synthesis of 1,2,3-triazoles from enolizable ketones, primary alkylamines, and 4-nitrophenyl azide. Synthesis 2017, 49, 4191–4198. [Google Scholar] [CrossRef]
- Duan, H.; Sengupta, S.; Petersen, J.L.; Akhmedov, N.G.; Shi, X. Triazole-Au(I) complexes: A new class of catalysts with improved thermal stability and reactivity for intermolecular alkyne hydroamination. J. Am. Chem. Soc. 2009, 131, 12100–12102. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Atoian, E.M.; Philippova, A.N.; Topchiy, M.A.; Asachenko, A.F.; Osipov, S.N. One-pot synthesis of 5-amino-1,2,3-triazole derivatives via dipolar azide–nitrile cycloaddition and Dimroth rearrangement under solvent-free conditions. Eur. J. Org. Chem. 2021, 2021, 1378–1384. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Topchiy, M.A.; Karsakova, I.V.; Chesnokov, G.A.; Smirnov, A.Y.; Minaeva, L.I.; Asachenko, A.F.; Nechaev, M.S. General method for the synthesis of 1,4-disubstituted 5-halo-1,2,3-triazoles. Eur. J. Org. Chem. 2017, 2017, 5225–5230. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Farahat, A.A.; El-Hiti, G.A. Synthesis and structure determination of 1-(4-methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2022, 2022, M1374. [Google Scholar] [CrossRef]
- Gökce, H.; Şen, F.; Sert, Y.; Abdel-Wahab, B.F.; Kariuki, B.M.; El-Hiti, G.A. Quantum computational investigation of (E)-1-(4-methoxyphenyl)-5-methyl-N′-(3-phenoxybenzylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molecules 2022, 27, 2193. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, B.M.; Abdel-Wahab, B.F.; El-Hiti, G.A. Synthesis and structural characterization of isostructural 4-(4-aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals 2021, 11, 795. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; El-Hiti, G.A. Synthesis and structure determination of 2-cyano-3-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)acrylamide. Molbank 2022, 2022, M1372. [Google Scholar] [CrossRef]
- Kamalraj, V.R.; Senthil, S.; Kannan, P. One-pot synthesis and the fluorescent behavior of 4-acetyl-5-methyl-1,2,3-triazole regioisomers. J. Mol. Struct. 2008, 892, 210–215. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
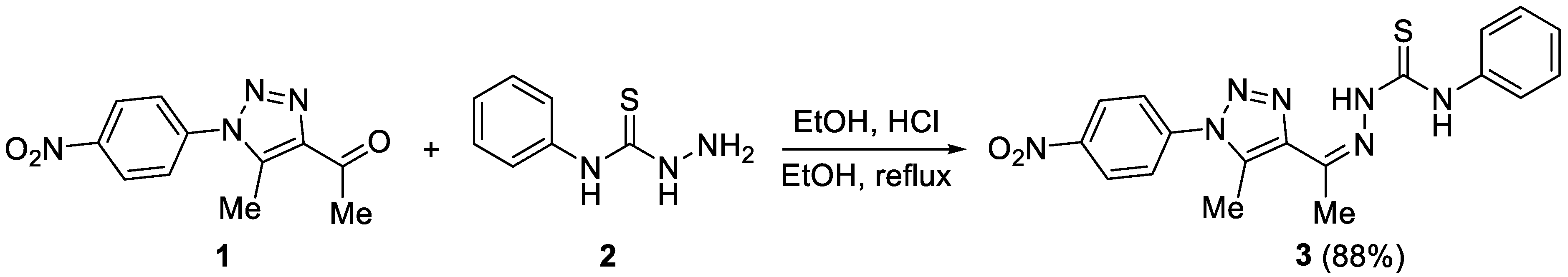

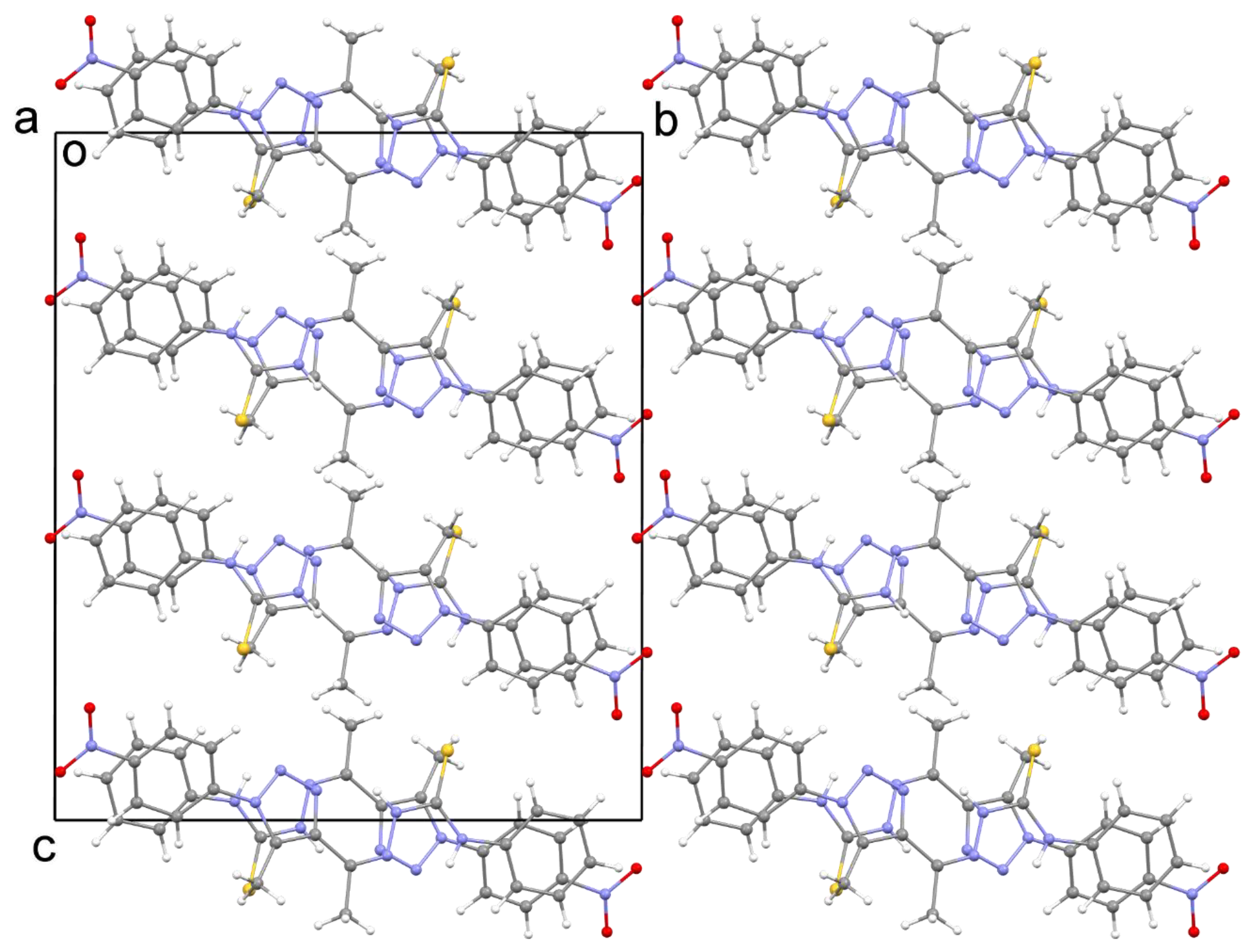
| Molecule | Distance (Å) | Angle (°) | ||
|---|---|---|---|---|
| M1 | N1…N3 | 2.542(2) | N1-H1…N3 | 112.3 |
| N2…N4 | 2.707(2) | N2-H2A…N4 | 132.5 | |
| M2 | N8…N10 | 2.546(2) | N8-H8…N10 | 113.0 |
| N9…N11 | 2.673(2) | N9-H9…N11 | 133.2 | |
| M3 | N15…N17 | 2.551(2 | N15-H15A…N17 | 114.2 |
| N16…N18 | 2.685(2) | N16-H16…N18 | 133.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, B.M.; Abdel-Wahab, B.F.; Bekheit, M.S.; El-Hiti, G.A. (Z)-2-(1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethylidene)-N-phenylhydrazine-1-carbothioamide. Molbank 2022, 2022, M1462. https://doi.org/10.3390/M1462
Kariuki BM, Abdel-Wahab BF, Bekheit MS, El-Hiti GA. (Z)-2-(1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethylidene)-N-phenylhydrazine-1-carbothioamide. Molbank. 2022; 2022(4):M1462. https://doi.org/10.3390/M1462
Chicago/Turabian StyleKariuki, Benson M., Bakr F. Abdel-Wahab, Mohamed S. Bekheit, and Gamal A. El-Hiti. 2022. "(Z)-2-(1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethylidene)-N-phenylhydrazine-1-carbothioamide" Molbank 2022, no. 4: M1462. https://doi.org/10.3390/M1462
APA StyleKariuki, B. M., Abdel-Wahab, B. F., Bekheit, M. S., & El-Hiti, G. A. (2022). (Z)-2-(1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethylidene)-N-phenylhydrazine-1-carbothioamide. Molbank, 2022(4), M1462. https://doi.org/10.3390/M1462








