N-(1-azido-2-(azidomethyl)butan-2-yl)-4-methylbenzenesulfonamide
Abstract
:1. Introduction
2. Results
3. Materials and Methods
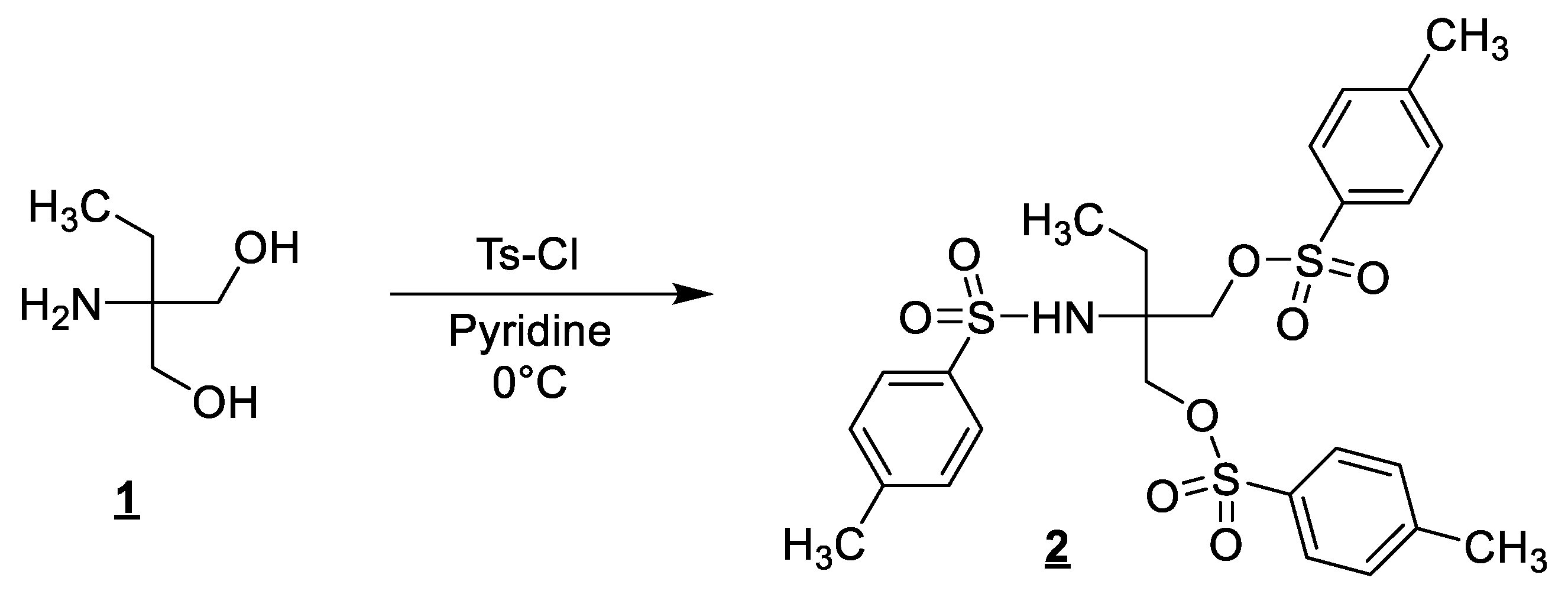
3.1. Synthesis of 2-Ethyl-2-((4-methylphenyl)sulfonamido)propane-1,3-diyl bis(4-methylbenzenesulfonate) (2)
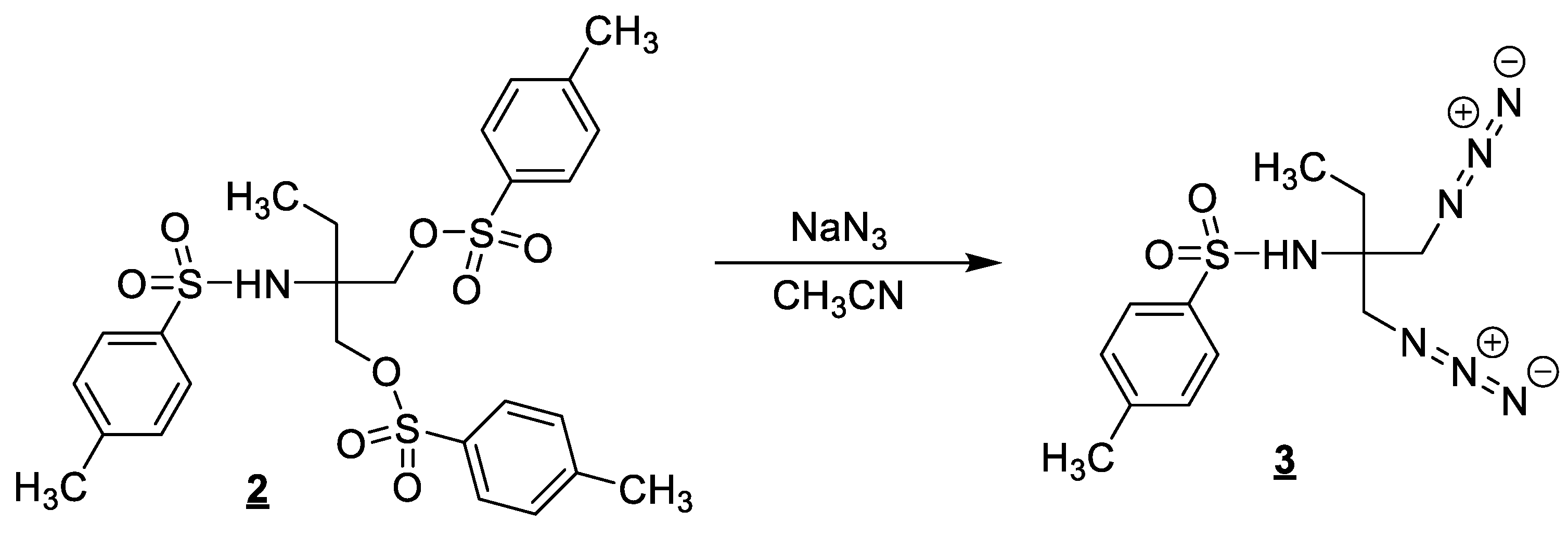
3.2. Synthesis of N-(1-azido-2-(azidomethyl)butan-2-yl)-4-methylbenzenesulfonamide (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawood, K.M.; Abdel-Wahab, B.F.; Raslan, M.A. Synthesis and applications of bi- and bis-triazole systems. Arkivoc 2018, part i, 179–215. [Google Scholar] [CrossRef]
- Dwivedi, A.R.; Kumar, V.; Yadav, R.P.; Kumar, N.; Jangid, K.; Anand, P.; Sharma, D.K.; Barnawal, S.; Kumar, V. Design, synthesis and evaluation of 4-phenyl-1,2,3-triazole substituted pyrimidine derivatives as antiproliferative and tubulin polymerization inhibitors. J. Mol. Struct. 2022, 1267, 133592. [Google Scholar] [CrossRef]
- Sadeghian, S.; Emami, L.; Mojaddami, A.; Khabnadideh, S.; Faghih, Z.; Zomorodian, K.; Rashidi, M.; Rezaei, Z. 1,2,4-Triazole derivatives as novel and potent antifungal agents: Design, synthesis and biological evaluation. J. Mol. Struct. 2022, 1271, 134039. [Google Scholar] [CrossRef]
- Mohamed, M.A.A.; Abd Allah, O.A.; Bekhit, A.A.; Kadry, A.M.; El-Saghier, A.M.M. Synthesis and antidiabetic activity of novel triazole derivatives containing amino acids. J. Heterocycl. Chem. 2020, 57, 2365–2378. [Google Scholar] [CrossRef]
- Singh, G.; Singh, J.; Singh, A.; Singh, J.; Kumar, M.; Gupta, K.; Chhibber, S. Synthesis, characterization and antibacterial studies of schiff based 1,2,3-triazole bridged silatranes. J. Organomet. Chem. 2018, 871, 21–27. [Google Scholar] [CrossRef]
- Slaihim, M.M.; Al-Suede, F.S.R.; Khairuddean, M.; Khadeer Ahamed, M.B.; Shah Abdul Majid, A.M. Synthesis, characterization of new derivatives with mono ring system of 1,2,4-triazole scaffold and their anticancer activities. J. Mol. Struct. 2019, 1196, 78–87. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.M.; Alvarez, L.X.; Escarpini dos Santos, N.; Barrios, A.C.M.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Aouine, Y.; Faraj, H.; Alami, A.; El Hallaoui, A.; Elachqar, A.; Kerbal, A. Simple and efficient synthesis of racemic 2-(tert-butoxycarbon-ylamino)-2-methyl-3-(1H-1,2,4- triazol-1-yl)propanoic acid, a new derivative of β-(1,2,4-triazol-1-yl)alanine. Molecules 2011, 16, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Atmani, A.; El Hallaoui, A.; El Hajji, S.; Roumestant, M.L.; Viallefont, P. From oxazolines to precursors of amino acids. Synth. Commun. 1991, 21, 2383–2390. [Google Scholar] [CrossRef]
- Aouine, Y.; Faraj, H.; Alami, A.; El Hallaoui, A.; EL Hajji, S.; Labriti, B.; Kerbal, A. Triheterocyclic compounds, oxazolinic precursors of biheterocyclic amino acids, part II: Phenothiazine derivatives and structural study of regioisomers through 1H-15N 2D NMR HMBC. J. Mar. Chim. Heterocycl. 2014, 13, 39–47. [Google Scholar] [CrossRef]
- Aouine, Y.; El Hallaoui, A.; Alami, A. N,N-Dibenzyl-1-(1-[(4-methyl-2-phenyl-4,5-dihydrooxazol-4-yl)methyl)]-1H-1,2,3-triazol-4-yl)methanamine. Molbank 2014, 2014, M819. [Google Scholar] [CrossRef]
- Hajib, S.; Alami, A.; Faraj, H.; Aouine, Y. 4-[(3,5-Dimethyl-1H-pyrazol-1-yl)methyl]-4-methyl-2-phenyl-4,5-dihydrooxazole. Molbank 2019, 2019, M1074. [Google Scholar] [CrossRef]
- Dioukhane, K.; Alami, A.; Aouine, Y.; El Omari, M.; El Ammari, L.; Saadi, M.; Assani, A.; Ouarsal, R. Synthesis, crystal structure and IR spectrum studies of 2-(4-methyl-2- phenyl-4,5-dihydro-oxazol-4-ylmethyl)- isoindole-1,3-dione. Mediterr. J. Chem. 2019, 9, 116–124. [Google Scholar] [CrossRef]
- Achamlale, S.; Alami, A.; Aouine, Y. Structure assignment of N-protected 2-(1H-1,2,3-triazol-1-yl)-glycine derivatives by chemical and spectroscopic methods. J. Mar. Chim. Heterocycl. 2019, 18, 61–69. [Google Scholar] [CrossRef]

| Position | δH | δC | Correlation H-H | Correlation C-H |
|---|---|---|---|---|
| 1 | - | 60.4 | - | - |
| 2 | 1.65 (2H, q, J = 7.5) | 24.5 | 2H2-2H2 | C2-2H2 |
| 3 | 0.59 (3H, t, J = 7.5) | 6.8 | 3H3-3H3 | C3-3H3 |
| 4; 4′ | 3.97 (2 × 2H, s) | 69.0 | 2H4-2H4 2H4′-2H4′ | C4-2H4 C4′-2H4′ |
| 5 | 5.08 (1H, s) | - | - | - |
| 6 | - | 143.7 | - | - |
| 7; 7′; 8; 8′ | 7.24–7.27; 7.66–7.69 (4 × -CHarom, 2d, J = 8.1) | 126.8–130.0 | 1H7-1H8 1H7′-1H8′ | C7-1H7; C7-1H7 C7′-1H7′; C8′-1H8′ |
| 9 | - | 139.1 | - | - |
| 10 | 2.42 (3H, s) | 21.6 | 3H10-3H10 | C10-3H10 |
| 11; 11′ | - | 145.4 | - | - |
| 12–15; 12′–15′ | 7.33–7.36; 7.68–7.71 (8 × -CHarom, 2d, J = 8.1) | 126.8–130.0 | 1H12-1H13 1H12′-1H13′ 1H14-1H15 1H14′-1H15′ | C12-1H12; C13-1H13 C14-1H14; C15-1H15 C12′-1H12′; C13′-1H13′ C14′-1H14′; C15′-1H15′ |
| 16–16′ | - | 131.9 | - | - |
| 17–17′ | 2.46 (2 × 3H, s) | 21.7 | 3H17-3H17 3H17′-3H17′ | C17-3H17 C17′-3H17′ |
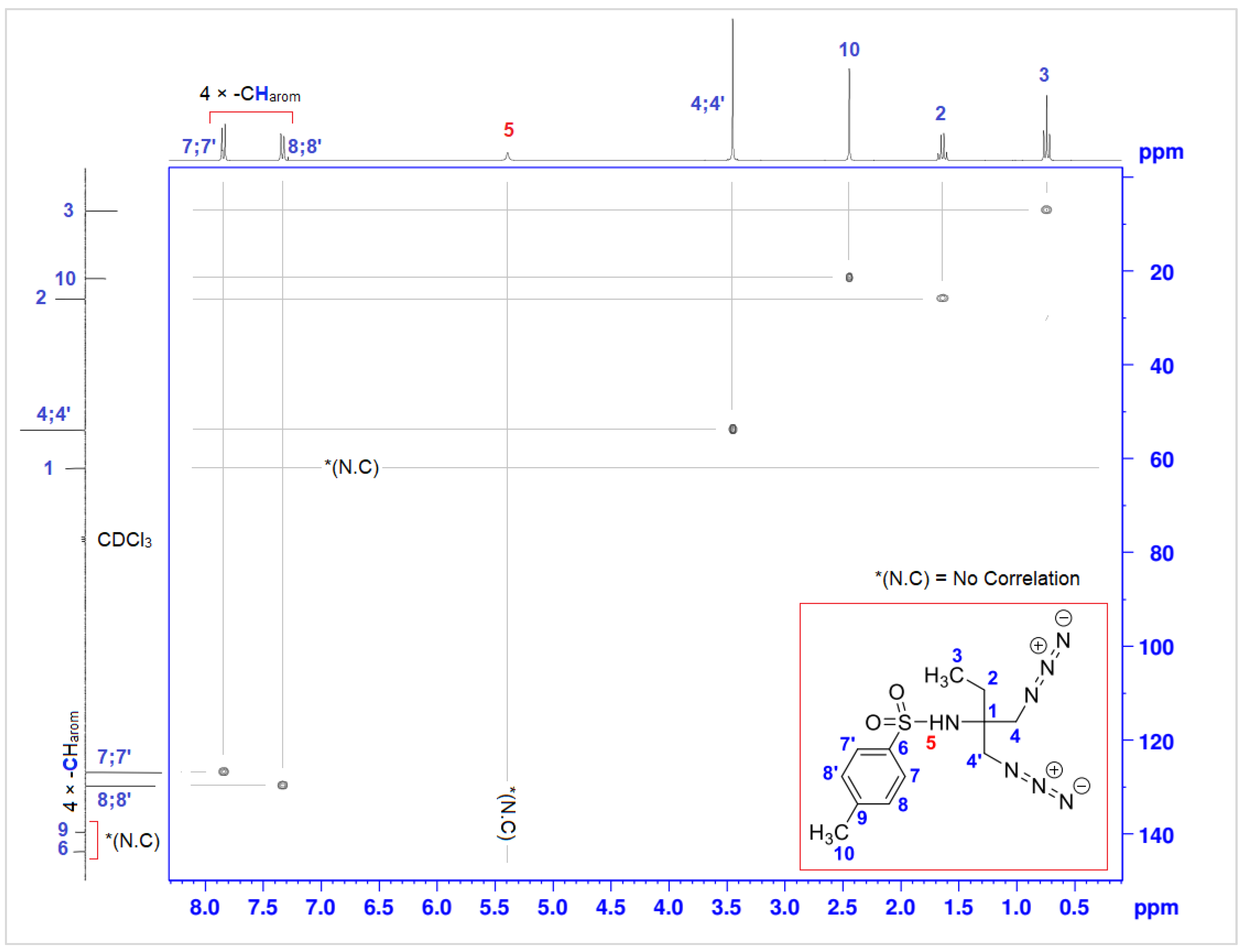
| Position | δH | δC | Correlation H-H | Correlation C-H |
|---|---|---|---|---|
| 1 | - | 62.0 | - | - |
| 2 | 1.64 (2H, q, J = 7.5) | 25.9 | 2H2-2H2 | C2-2H2 |
| 3 | 0.74 (3H, t, J = 7.5) | 7.1 | 3H3-3H3 | C3-3H3 |
| 4; 4′ | 3.45 (2 × 2H, s) | 53.9 | 2H4-2H4 2H4′-2H4′ | C4-2H4 C4′-2H4′ |
| 5 | 5.39 (1H, s) | - | - | - |
| 6 | - | 143.7 | - | - |
| 7; 7′ | 7.82–7.85 (2 × -CHarom, d, J = 8.1) | 126.8 | 1H7-1H8 1H7′-1H8′ | C7-1H7 C7′-1H7′ |
| 8; 8′ | 7.31–7.34 (2 × -CHarom, d, J = 8.1) | 129.8 | 1H8-1H7 1H8′-1H7′ | C8-1H8 C8′-1H8′ |
| 9 | - | 139.6 | - | - |
| 10 | 2.44 (3H, s) | 21.5 | 3H10-3H10 | C10-3H10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajib, S.; Aouine, Y.; Faraj, H.; Alami, A. N-(1-azido-2-(azidomethyl)butan-2-yl)-4-methylbenzenesulfonamide. Molbank 2022, 2022, M1448. https://doi.org/10.3390/M1448
Hajib S, Aouine Y, Faraj H, Alami A. N-(1-azido-2-(azidomethyl)butan-2-yl)-4-methylbenzenesulfonamide. Molbank. 2022; 2022(3):M1448. https://doi.org/10.3390/M1448
Chicago/Turabian StyleHajib, Sara, Younas Aouine, Hassane Faraj, and Anouar Alami. 2022. "N-(1-azido-2-(azidomethyl)butan-2-yl)-4-methylbenzenesulfonamide" Molbank 2022, no. 3: M1448. https://doi.org/10.3390/M1448
APA StyleHajib, S., Aouine, Y., Faraj, H., & Alami, A. (2022). N-(1-azido-2-(azidomethyl)butan-2-yl)-4-methylbenzenesulfonamide. Molbank, 2022(3), M1448. https://doi.org/10.3390/M1448






