[N,N′-Di-tert-butyl-P,P-diphenylphosphinimidic Amidato-κN,κN′]chlorosilicon-κSi-tetracarbonyliron
Abstract
:1. Introduction
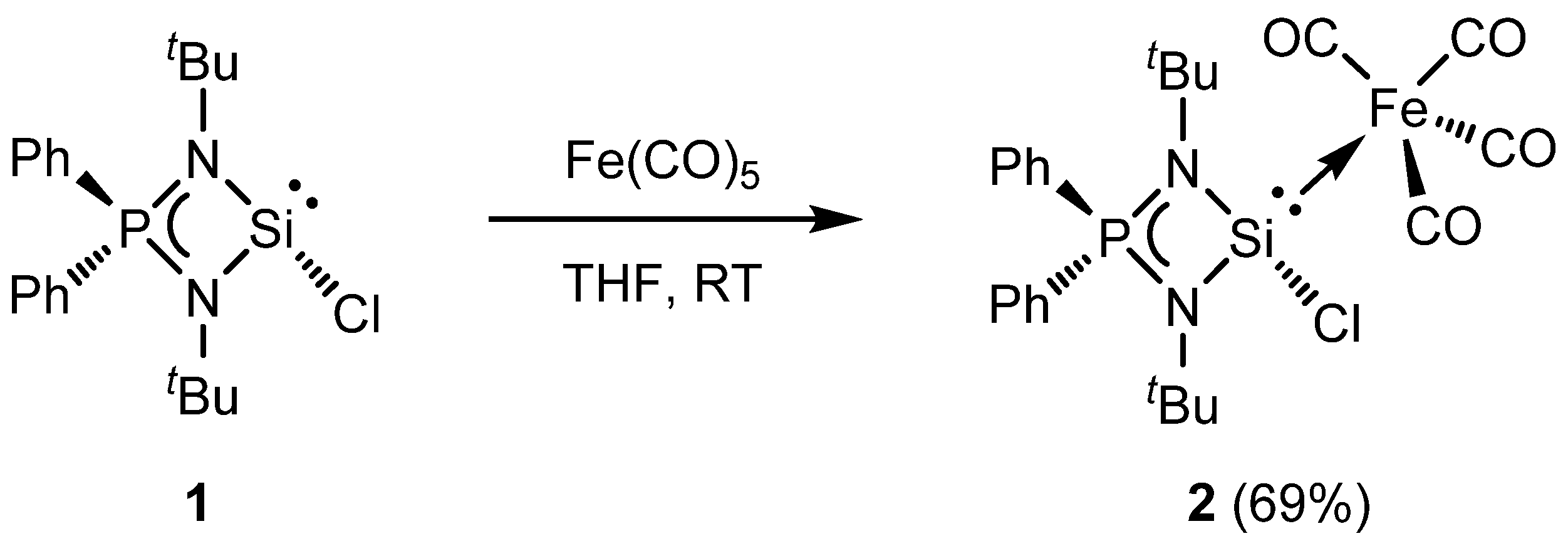
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of Complex 2
3.3. SCXRD Analysis of 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagendran, S.; Roesky, H.W. The chemistry of aluminum(I), silicon(II), and germanium(II). Organometallics 2008, 27, 457–492. [Google Scholar] [CrossRef]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable heavier carbene analogues. Chem. Rev. 2009, 109, 3479–3511. [Google Scholar] [CrossRef] [PubMed]
- Krahfuss, M.J.; Radius, U. N-Heterocyclic silylenes as ambiphilic activators and ligands. Dalton Trans. 2021, 50, 6752–6765. [Google Scholar] [CrossRef]
- Blom, B.; Stoelzel, M.; Driess, M. New vistas in N-heterocyclic silylene (NHSi) transition-metal coordination chemistry: Syntheses, structures and reactivity towards activation of small molecules. Chem. Eur. J. 2013, 19, 40–62. [Google Scholar] [CrossRef] [PubMed]
- Raoufmoghaddam, S.; Zhou, Y.-P.; Wang, Y.; Driess, M. N-heterocyclic silylene as powerful steering ligands in catalyst. J. Organomet. Chem. 2017, 829, 2–10. [Google Scholar] [CrossRef]
- Zhou, Y.-P.; Driess, M. Isolable silylene ligands can boost efficiencies and selectivities in metal-mediated catalysis. Angew. Chem. Int. Ed. 2019, 58, 3715–3728. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, L.; Cabeza, J.A.; García-Álvarez, P.; Polo, D. The transition-metal chemistry of amidinatosilylenes, -germylenes and -stannylenes. Coord. Chem. Rev. 2015, 300, 1–28. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Y.; Sun, H.; Li, X. Progress in the preparation and characterization of silylene iron, cobalt and nickel complexes. Dalton. Trans. 2021, 50, 6766–6772. [Google Scholar] [CrossRef]
- Wang, Y.; Koestenko, A.; Yao, S.; Driess, M. Divalent silicon-assisted activation of dihydrogen in a bis(N-heterocyclic silylene)xantene nickel(0) complex for efficient catalytic hydrogenation of olefins. J. Am. Chem. Soc. 2017, 139, 13499–13506. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Cui, C. An arene-tethered silylene ligand enabling reversible dinitrogen binding to iron and catalytic silylation. Chem. Commun. 2018, 54, 8124–8127. [Google Scholar] [CrossRef]
- Blom, B.; Enthaler, S.; Inoue, S.; Irran, E.; Driess, M. Electron-rich N-heterocyclic silylene (NHS)-iron complexes: Synthesis, structures, and catalytic ability of an isolable hydridosilylene-iron complex. J. Am. Chem. Soc. 2013, 135, 6703–6713. [Google Scholar] [CrossRef]
- Gallego, D.; Inoue, S.; Blom, B.; Driess, M. Highly electron-rich pincer-type iron complexes bearing innocent bis(metallylene)pyridine ligands: Syntheses, structures, and catalytic activity. Organometallics 2014, 33, 6885–6897. [Google Scholar] [CrossRef]
- Luecke, M.-P.; Porwal, D.; Kostenko, A.; Zhou, Y.-P.; Yao, S.; Keck, M.; Limberg, C.; Oestreich, M.; Driess, M. Bis(silylenyl)-substituted ferrocene-stabilized h6-arene iron(0) complexes: Synthesis, structure and catalytic application. Dalton. Trans. 2017, 46, 16412–16418. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xue, X.; Liu, Y.; Yu, N.; Krogman, J.P. Aminolysis of bis[bis(trimethylsilyl)amido]-manganese, -iron, and -cobalt for the synthesis of mono- and bis- silylene complexes. Dalton. Trans. 2020, 49, 12586–12591. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qi, X.; Li, K.; Li, X.; Sun, H.; Fuhr, O.; Fenske, D. (NHSi) iron (II) hydride for hydrosilylation of aldehydes and ketones. Appl. Organomet. Chem. 2021, 35, e6286. [Google Scholar] [CrossRef]
- Fan, Q.; Li, Q.; Qi, X.; Du, X.; Ren, S.; Li, X.; Fuhr, O.; Sun, H. Synthesis and structure of silylene iron complex. Z. Anorg. Allg. Chem. 2022, 648, e202200084. [Google Scholar] [CrossRef]
- Yang, W.; Fu, H.; Wang, H.; Chen, M.; Ding, Y.; Roesky, H.W.; Jana, A. A base-stabilized silylene with tricoordinate silicon atom as a ligand for a metal complex. Inorg. Chem. 2009, 48, 5058–5060. [Google Scholar] [CrossRef]
- Tacke, R.; Kobelt, C.; Baus, J.A.; Bertermann, R.; Burschka, C. Synthesis, structure and reactivity of a donor-stabilized silylene with a bulky bidentate benzamidinato ligand. Dalton Trans. 2015, 44, 14959–14974. [Google Scholar] [CrossRef]
- Breit, N.C.; Eisenhut, C.; Inoue, S. Phosphinosilylene as a novel ligand system for heterobimetallic complexes. Chem. Commun. 2016, 52, 5523–5526. [Google Scholar] [CrossRef]
- Blom, B.; Pohl, M.; Tan, G.; Gallego, D.; Driess, M. From unsymmetrically substituted benzamidinato and guanidinato dichlorohydridosilanes to novel hydrido N-heterocyclic silylene iron complexes. Organometallics 2014, 33, 5272–5282. [Google Scholar] [CrossRef]
- Takahashi, S.; Sekiguchi, J.; Ishii, A.; Nakata, N. An iminophosphonamido-chlorosilylene as a strong σ-donating NHSi ligand: Synthesis and coordination chemistry. Angew. Chem. Int. Ed. 2021, 133, 4101–4105. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishii, A.; Nakata, N. Interconversion between a silaimine and an aminosilylene supported by an iminophosphonamide ligand. Chem. Commun. 2021, 57, 3203–3206. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ishii, A.; Nakata, N. Formation of silaimines from a sterically demanding iminophosphonamido chlorosilylene via intramolecular N–P bond cleavage. Chem. Commun. 2021, 57, 6728–6731. [Google Scholar] [CrossRef]
- Nakaya, K.; Takahashi, S.; Ishii, A.; Boonpalit, K.; Surawatanawong, P.; Nakata, N. Hydroboration of carbonyls and imines by an iminophosphonamido Tin(II) precatalyst. Dalton Trans. 2021, 50, 14810–14819. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, K.; Ishii, A.; Nakata, N. Aluminum(III) di- and monochlorides incorporating an N,N’-chelating iminophosphonamide ligand: Synthesis and structures. Mendeleev Commun. 2022, 32, 71–73. [Google Scholar] [CrossRef]
- Takahashi, S.; Sekiguchi, J.; Nakaya, K.; Ishii, A.; Nakata, N. Halogen-exchange reactions of iminophosphonamido-chlorosilylenes with alkali halides: Convenient synthesis of heavier halosilylenes. Inorg. Chem. 2022, 61, 7266–7273. [Google Scholar] [CrossRef]
- Klein, A.; Neugebauer, M.; Krest, A.; Lüning, A.; Garbe, S.; Arefyeva, N.; Schlörer, N. Five coordinate platinum(II) in [Pt(Bpy)(Cod)(Me)][SbF6]: A structural and spectroscopic study. Inorganics 2015, 3, 118–138. [Google Scholar] [CrossRef]
- Burnett, M.N.; Johnson, C.K. ORTEPIII; Report ORNL-6895; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
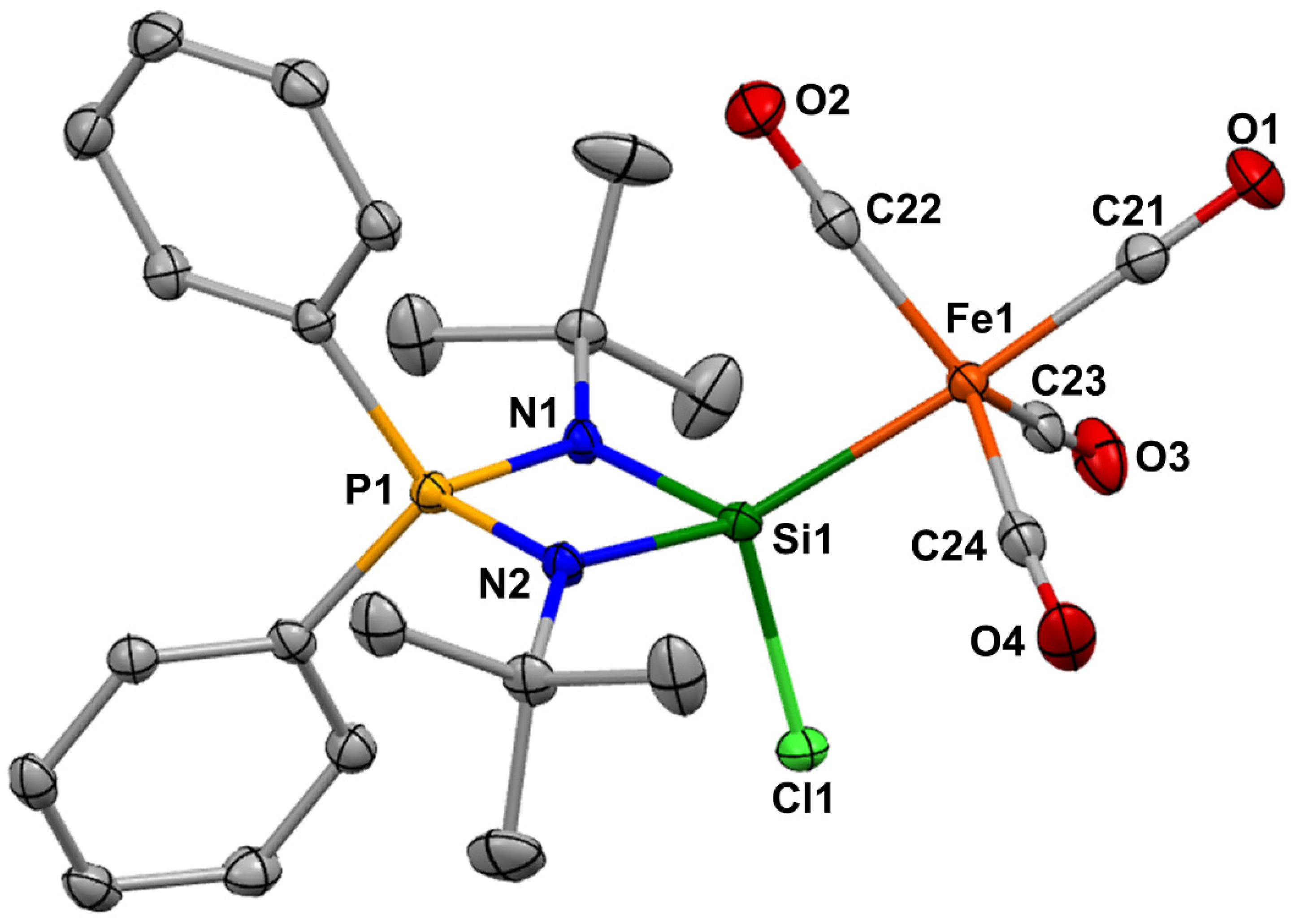
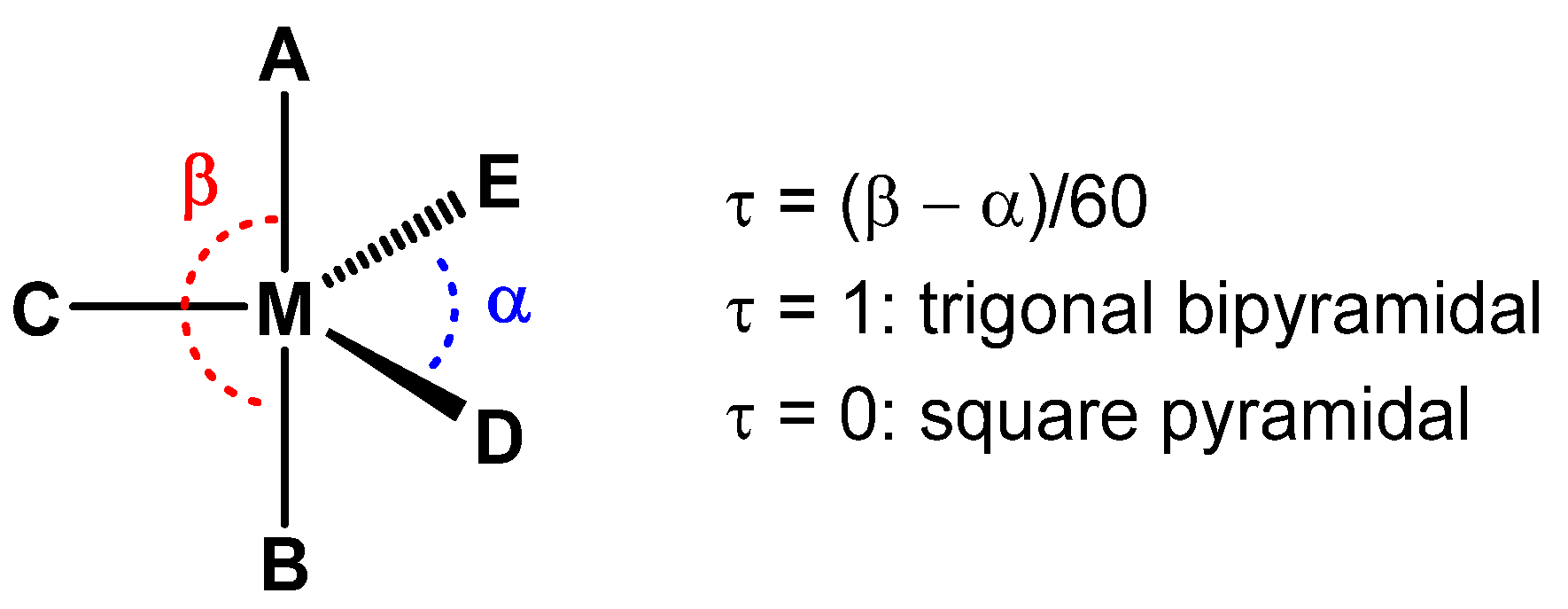
| Bond Lengths | [Å] | Bond Angles | [°] |
|---|---|---|---|
| Si1–Fe1 | 2.2675(6) | Si1–Fe1–C21 | 174.28(6) |
| Si1–Cl1 | 2.1161(7) | Si1–Fe1–C22 | 86.99(6) |
| Si1–N1 | 1.8001(15) | Si1–Fe1–C23 | 86.12(6) |
| Si1–N2 | 1.8078(15) | Si1–Fe1–C24 | 91.12(6) |
| Fe1–C21 | 1.791(2) | C22–Fe1–C23 | 127.39(9) |
| Fe1–C22 | 1.784(2) | C23–Fe1–C24 | 111.64(9) |
| Fe1–C23 | 1.774(2) | C22–Fe1–C24 | 120.59(9) |
| Fe1–C24 | 1.788(2) | Cl1–Si1–N1 | 104.37(6) |
| Cl1–Si1–N2 | 104.82(5) | ||
| N1–Si1–N2 | 80.31(7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, S.; Nakaya, K.; Ishii, A.; Nakata, N. [N,N′-Di-tert-butyl-P,P-diphenylphosphinimidic Amidato-κN,κN′]chlorosilicon-κSi-tetracarbonyliron. Molbank 2022, 2022, M1433. https://doi.org/10.3390/M1433
Takahashi S, Nakaya K, Ishii A, Nakata N. [N,N′-Di-tert-butyl-P,P-diphenylphosphinimidic Amidato-κN,κN′]chlorosilicon-κSi-tetracarbonyliron. Molbank. 2022; 2022(3):M1433. https://doi.org/10.3390/M1433
Chicago/Turabian StyleTakahashi, Shintaro, Kazuki Nakaya, Akihiko Ishii, and Norio Nakata. 2022. "[N,N′-Di-tert-butyl-P,P-diphenylphosphinimidic Amidato-κN,κN′]chlorosilicon-κSi-tetracarbonyliron" Molbank 2022, no. 3: M1433. https://doi.org/10.3390/M1433
APA StyleTakahashi, S., Nakaya, K., Ishii, A., & Nakata, N. (2022). [N,N′-Di-tert-butyl-P,P-diphenylphosphinimidic Amidato-κN,κN′]chlorosilicon-κSi-tetracarbonyliron. Molbank, 2022(3), M1433. https://doi.org/10.3390/M1433






