Methyl 2-((3-(3-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl)thio)acetate
Abstract
:1. Introduction
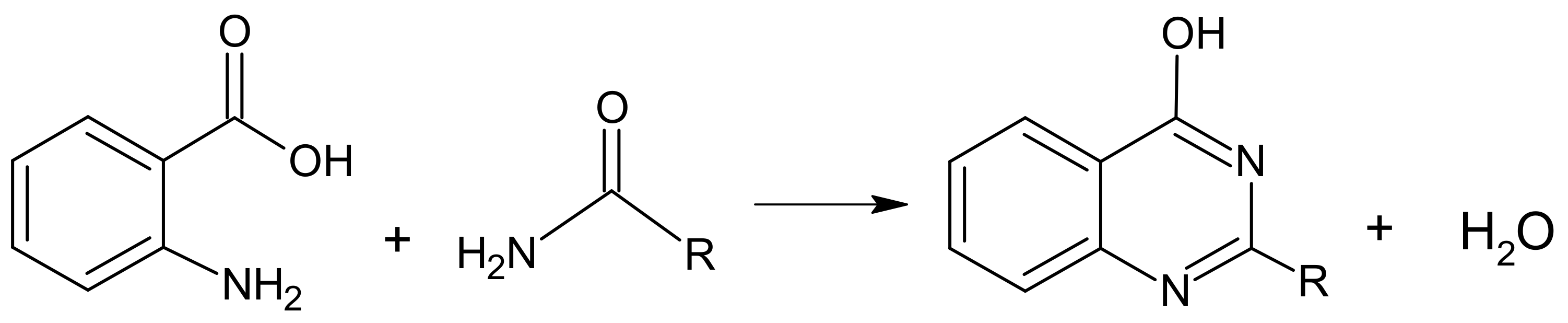
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayyed, M.A.; Mokle, S.S.; Vibhute, Y.B. Synthesis of 6-iodo/bromo- 3-amino-2-methylquinazolin-4 (3H)-ones by direct halogenation and their Schiff base derivatives. Arkivoc 2006, 2006, 221–226. [Google Scholar] [CrossRef]
- Hassanzadeh, F.; Jafari, E.; Hakimelahi, G.; Khajouei, M.R.; Jalali, M.; Khodarahmi, G. Antibacterial, antifungal and cytotoxic evaluation of some new quinazolinone derivatives. Res. Pharm. Sci. 2012, 7, 87–94. [Google Scholar]
- Selvam, P.; Vijayalakshimi, P.; Smee, D.F.; Gowen, B.B.; Julander, J.G.; Day, C.W.; Barnard, D.L. Novel 3-sulphonamido-quinazolin-4(3H)-One Derivatives: Microwave-Assisted Synthesis and Evaluation of Antiviral Activities against Respiratory and Biodefense Viruses. Antivir. Chem. Chemother. 2007, 18, 301–305. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Ayyad, R.R.; Shawer, T.Z.; Abdel-Aziz, A.A.-M.; El-Azab, A.S. Synthesis and Antitumor Evaluation of Trimethoxyanilides Based on 4(3H)-Quinazolinone Scaffolds. Eur. J. Med. Chem. 2016, 112, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Gawad, N.M.A.; Georgey, H.H.; Youssef, R.M.; El-Sayed, N.A. Synthesis and Antitumor Activity of Some 2, 3-Disubstituted Quinazolin-4(3H)-Ones and 4, 6- Disubstituted-1, 2, 3, 4-Tetrahydroquinazolin-2H-Ones. Eur. J. Med. Chem. 2010, 45, 6058–6067. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H.M.; Bhardwaj, V.; Chauhan, A. Synthesis and in Vitro Antitumor Activity of Substituted Quinazoline and Quinoxaline Derivatives: Search for Anticancer Agent. Eur. J. Med. Chem. 2011, 46, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- El-Subbagh, H.I.; Sabry, M.A. 2-Substituted-Mercapto-Quinazolin-4(3H)-Ones as DHFR Inhibitors. Mini Rev. Med. Chem. 2021, 21, 2249–2260. [Google Scholar] [CrossRef]
- Petrov, K.G.; Zhang, Y.-M.; Carter, M.; Cockerill, G.S.; Dickerson, S.; Gauthier, C.A.; Guo, Y.; Mook, R.A.; Rusnak, D.W.; Walker, A.L.; et al. Optimization and SAR for Dual ErbB-1/ErbB-2 Tyrosine Kinase Inhibition in the 6-Furanylquinazoline Series. Bioorg. Med. Chem. Lett. 2006, 16, 4686–4691. [Google Scholar] [CrossRef]
- Rosenthal, A.S.; Tanega, C.; Shen, M.; Mott, B.T.; Bougie, J.M.; Nguyen, D.-T.; Misteli, T.; Auld, D.S.; Maloney, D.J.; Thomas, C.J. Potent and Selective Small Molecule Inhibitors of Specific Isoforms of Cdc2-like Kinases (Clk) and Dual Specificity Tyrosine-Phosphorylation-Regulated Kinases (Dyrk). Bioorg. Med. Chem. Lett. 2011, 21, 3152–3158. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis, Antibacterial, Antifungal and Anti-HIV Evaluation of Schiff and Mannich Bases of Isatin Derivatives with 3-Amino-2-Methylmercapto Quinazolin-4(3H)-One. Pharm. Acta Helv. 1999, 74, 11–17. [Google Scholar] [CrossRef]
- Safakish, M.; Hajimahdi, Z.; Aghasadeghi, M.R.; Vahabpour, R.; Zarghi, A. Design, Synthesis, Molecular Modeling and Anti-HIV Assay of Novel Quinazolinone Incorporated Coumarin Derivatives. Curr. HIV Res. 2020, 18, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hajimahdi, Z.; Zabihollahi, R.; Aghasadeghi, M.R.; Zarghi, A. Design, Synthesis, Docking Studies and Biological Activities Novel 2,3- Diaryl-4-Quinazolinone Derivatives as Anti-HIV-1 Agents. Curr. HIV Res. 2019, 17, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Von Niementowski, S. Synthesen von Chinazolinverbindungen. J. Prakt. Chem. 1895, 51, 564–572. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X.-F. Recent Advances in 4(3H)-Quinazolinone Syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Kidwai, M.; Rastogi, S.; Mohan, R. A Novel Route to the Niementowski Reaction. Croat. Chem. Acta 2003, 76, 365–369. [Google Scholar] [CrossRef]
- Khajavi, M.S.; Afshani, P.; Rad Moghadam, K. Microwave Irradiation Promoted the Niementowski Reaction Preparation of Substituted Quinazolinones and Quinolines. Iran. J. Chem. Chem. Eng. 1998, 17, 29–32. [Google Scholar] [CrossRef]
- Alexandre, F.-R.; Berecibar, A.; Besson, T. Microwave-Assisted Niementowski Reaction. Back to the Roots. Tetrahedron Lett. 2002, 43, 3911–3913. [Google Scholar] [CrossRef]
- Alexandre, F.-R.; Berecibar, A.; Wrigglesworth, R.; Besson, T. Novel Series of 8H-Quinazolino[4,3-b]Quinazolin-8-Ones via Two Niementowski Condensations. Tetrahedron 2003, 59, 1413–1419. [Google Scholar] [CrossRef]
- Patil, S.; Jadhav, S.; Shejwal, R.V.; Deshmukh, M. Microwave-Assisted Cyclocondensation for the Synthesis of 3-Aryl-2-Thioquinazolin-4(3H)-Ones. Asian J. Chem. 2012, 24, 1858–1860. [Google Scholar]
- Ziarani, G.M.; Asl, Z.K.; Gholamzadeh, P.; Badiei, A.; Afshar, M. The Use of SrFe12O19 Magnetic Nanoparticles as an Efficient Catalyst in the Modified Niementowski Reaction. Appl. Organomet. Chem. 2017, 31, e3830. [Google Scholar] [CrossRef]
- Moussa, G.; Alaaeddine, R.; Alaeddine, L.M.; Nassra, R.; Belal, A.S.F.; Ismail, A.; El-Yazbi, A.F.; Abdel-Ghany, Y.S.; Hazzaa, A. Novel Click Modifiable Thioquinazolinones as Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation and Docking Study. Eur. J. Med. Chem. 2018, 144, 635–650. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S. Synthesis of Some New Substituted 2-Mercaptoquinazoline Analogs as Potential Antimicrobial Agents. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 333–348. [Google Scholar] [CrossRef]
- Al-Khuzaie, M.; Al-Majidi, S. Synthesis, Characterization and Evaluation Antimicrobial Activity of Some New Substituted 2-Mercapto-3-Phenyl-4(3H)-Quinazolinone. Iraqi J. Sci. 2014, 55, 582–593. [Google Scholar]
- Cong, N.T.; Nguyen, Q.; Huynh, P.D.; Nguyen, T.; Phuong, N.; Nguyen, H.-H. Synthesis and Cytotoxic Activity against K562 and MCF7 Cell Lines of Some N-(5-Arylidene-4-Oxo-2-Thioxothiazolidin-3-Yl)-2-((4-Oxo-3-Phenyl-3,4-Dihydroquinazoline-2-Yl)Thio)Acetamide Compounds. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Komar, M.; Kraljević, T.G.; Jerković, I.; Molnar, M. Application of Deep Eutectic Solvents in the Synthesis of Substituted 2-Mercaptoquinazolin-4(3H)-Ones: A Comparison of Selected Green Chemistry Methods. Molecules 2022, 27, 558. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.-M.; Bua, S.; Nocentini, A.; AlSaif, N.A.; Alanazi, M.M.; El-Gendy, M.A.; Ahmed, H.E.A.; Supuran, C.T. S-Substituted 2-Mercaptoquinazolin-4(3H)-One and 4-Ethylbenzensulfonamides Act as Potent and Selective Human Carbonic Anhydrase IX and XII Inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 733–743. [Google Scholar] [CrossRef]
- Khalil, A.K. Phase-Transfer Catalyzed Alkylation and Cycloalkylation of 2-Mercaptoquinazolin-4(3H)-One. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 2533–2541. [Google Scholar] [CrossRef]
- Molnar, M.; Klenkar, J.; Tarnai, T. Eco-Friendly Rapid Synthesis of 3-Substituted-2-Thioxo-2,3-Dihydroquinazolin-4(1H)-Ones in Choline Chloride Based Deep Eutectic Solvent. Synth. Commun. 2017, 47, 1040–1045. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelkovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Periš, I.; Komar, M. Choline Chloride Based Deep Eutectic Solvents as a Tuneable Medium for Synthesis of Coumarinyl 1,2,4-Triazoles: Effect of Solvent Type and Temperature. Eur. J. Org. Chem. 2019, 2019, 2688–2694. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, M.; Komar, M.; Jerković, I. Methyl 2-((3-(3-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl)thio)acetate. Molbank 2022, 2022, M1434. https://doi.org/10.3390/M1434
Molnar M, Komar M, Jerković I. Methyl 2-((3-(3-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl)thio)acetate. Molbank. 2022; 2022(3):M1434. https://doi.org/10.3390/M1434
Chicago/Turabian StyleMolnar, Maja, Mario Komar, and Igor Jerković. 2022. "Methyl 2-((3-(3-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl)thio)acetate" Molbank 2022, no. 3: M1434. https://doi.org/10.3390/M1434
APA StyleMolnar, M., Komar, M., & Jerković, I. (2022). Methyl 2-((3-(3-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl)thio)acetate. Molbank, 2022(3), M1434. https://doi.org/10.3390/M1434







