3,6-Dichlorobenzene-1,2,4,5-tetraol
Abstract
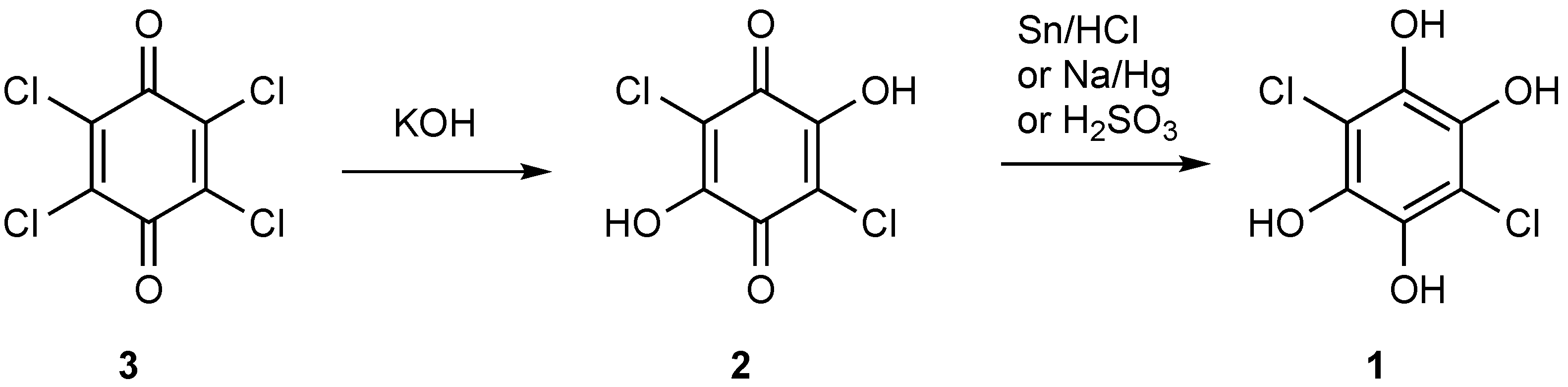
1. Introduction
2. Results
3. Experimental Section
3,6-Dichlorobenzene-1,2,4,5-tetraol (1)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Graebe, C. Untersuchungen über die Chinongruppe. Liebigs Ann. Chem. 1868, 146, 1–65. [Google Scholar] [CrossRef][Green Version]
- Senkbeil, H.O.; Brust, H.F. Neutral polychloro-aliphatic acid esters of polyhydroxyphenols. US Patent 2815365, 3 December 1957. [Google Scholar]
- Konopik, N.; Luf, W. Polarographische Untersuchung der Kinetik der Reaktion zwischen Germaniumsäure und 1,2,4,5-Tetrahydroxy-3,6-dichlorobenzol. Monatsh. Chem. 1972, 103, 355–366. [Google Scholar] [CrossRef]
- Yang, Z.; Gould, E.S. Reactions of 1,4-benzoquinones with s2 reducing centers. Dalton Trans. 2003, 2219–2223. [Google Scholar] [CrossRef]
- Yang, Z.; Gould, E.S. Reductions by aquatitanium(II). Dalton Trans. 2005, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.B.; Gould, E.S. Hypovalent titanium and Ti(II)–Ti(III) interconversions. Dalton Trans. 2010, 39, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; McCusker, J.K. Spin exchange effects on the physicochemical properties of tetraoxolene-bridged bimetallic complexes. Inorg. Chem. 2007, 46, 3257–3274. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hudson, T.A.; McCormick, L.J.; Robson, R. Coordination polymers of 2,5-dihydroxybenzoquinone and chloranilic acid with the (10,3)-a topology. Cryst. Growth Des. 2011, 11, 2717–2720. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Abrahams, B.F.; Auckett, J.E.; Chevreau, H.; Dharma, A.D.; Duyker, S.; He, Q.; Hua, C.; Hudson, T.A.; Murray, K.S.; et al. Square grid metal chloranilate networks as robust host systems for guest sorption. Chem. Eur. J. 2019, 25, 5222–5234. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, C.J.; Abrahams, B.F.; D’Alessandro, D.M.; Hudson, T.A.; Murase, R.; Robson, R.; White, K.F. Role of NEt4+ in orienting and locking together [M2lig3]2− (6,3) sheets (H2lig = chloranilic or fluoranilic acid) to generate spacious channels perpendicular to the sheets. Cryst. Growth Des. 2017, 17, 1465–1470. [Google Scholar] [CrossRef]
- van Koeverden, M.P.; Abrahams, B.F.; D’Alessandro, D.M.; Doheny, P.W.; Hua, C.; Hudson, T.A.; Jameson, G.N.L.; Murray, K.S.; Phonsri, W.; Robson, R.; et al. Tuning charge-state localization in a semiconductive iron(III)-chloanilate framework magnet using a redox-active cation. Chem. Mater. 2020, 32, 7551–7563. [Google Scholar] [CrossRef]
- van Koeverden, M.P.; Abrahams, B.F.; Hua, C.; Hudson, T.A.; Robson, R. Inducing structural diversity in anionic metal–tetraoxolene coordination polymers using templating methyl viologen countercations. Cryst. Growth Des. 2022, 22, 1319–1332. [Google Scholar] [CrossRef]
- Biliskov, N.; Kojic-Prodic, B.; Mali, G.; Molcanov, K.; Stare, J. A partial proton transfer in hydrogen bond O–H… in crystals of anhydrous potassium and rubidium complex chloranilates. J. Phys. Chem. A 2011, 115, 3154–3166. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, G.; Yathirajan, H.S.; Al-arique, D.N.M.H.; Narayana, B.; Kubicki, M. Chloranilic acid: A redetermination at 100 K. Acta Crystallogr. Sect. E 2010, 66, o497–o498. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, V.; Molcanov, K.; Jelsch, C.; Wenger, E.; Krawczuk, A.; Juric, M.; Dubraja, L.A.; Kojic-Prodic, B. Malleable electronic structure of chloranilic acid and its species determined by X-ray charge density studies. Cryst. Growth Des. 2019, 19, 2802–2810. [Google Scholar] [CrossRef]
- Sosa-Rivadeneyra, M.V.; Vasquez-Ríos, M.G.; Vargas-Olvera, E.C.; Mendoza, M.E.; Varela-Caselis, J.L.; Meza-León, R.L.; Sánchez-Guadarrama, M.O.; Höpfl, H. Crystal structures of organic salts of chloranilic acid and 2,2′-bi(3-hydroxy-1,4-naphthoquinone) acting as proton donors to 4,4′-bipyridine and 1,4-diazabicyclo [2.2.2]octane: 3D networks with bifurcated N+–H…O−/O or N+–H…O/Cl synthons. J. Mol. Struct. 2020, 1205, 127609. [Google Scholar] [CrossRef]
- Kabir, M.K.; Tobita, H.; Matsuo, H.; Nagayoshi, K.; Yamada, K.; Adachi, K.; Sugiyama, Y.; Kitagawa, S.; Kawata, S. Crystal engineering using the versatility of 2,5-dichloro-3,6-dihydroxy-1,4-benzoquinone with organic and metal complex partners. Cryst. Growth Des. 2003, 3, 791–798. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
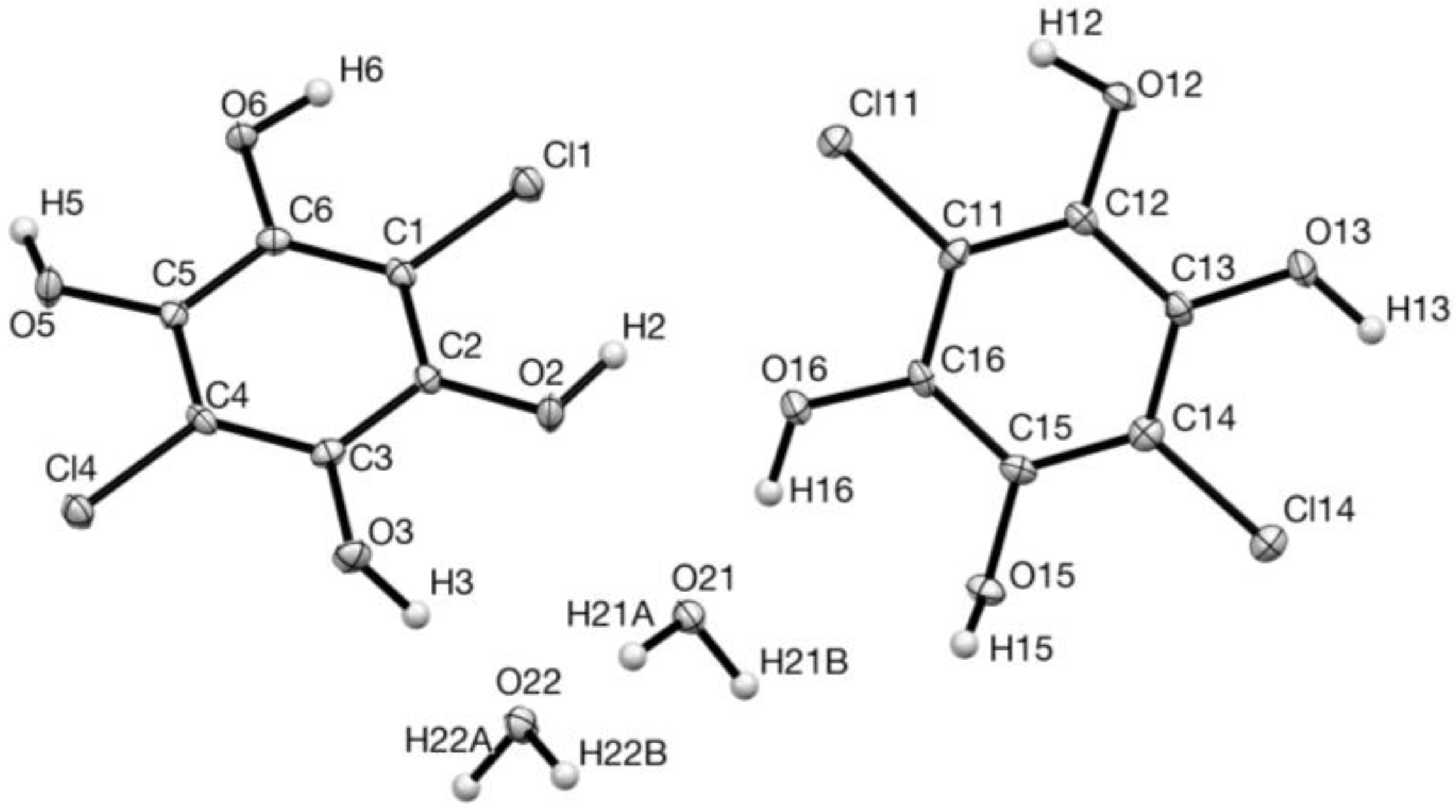
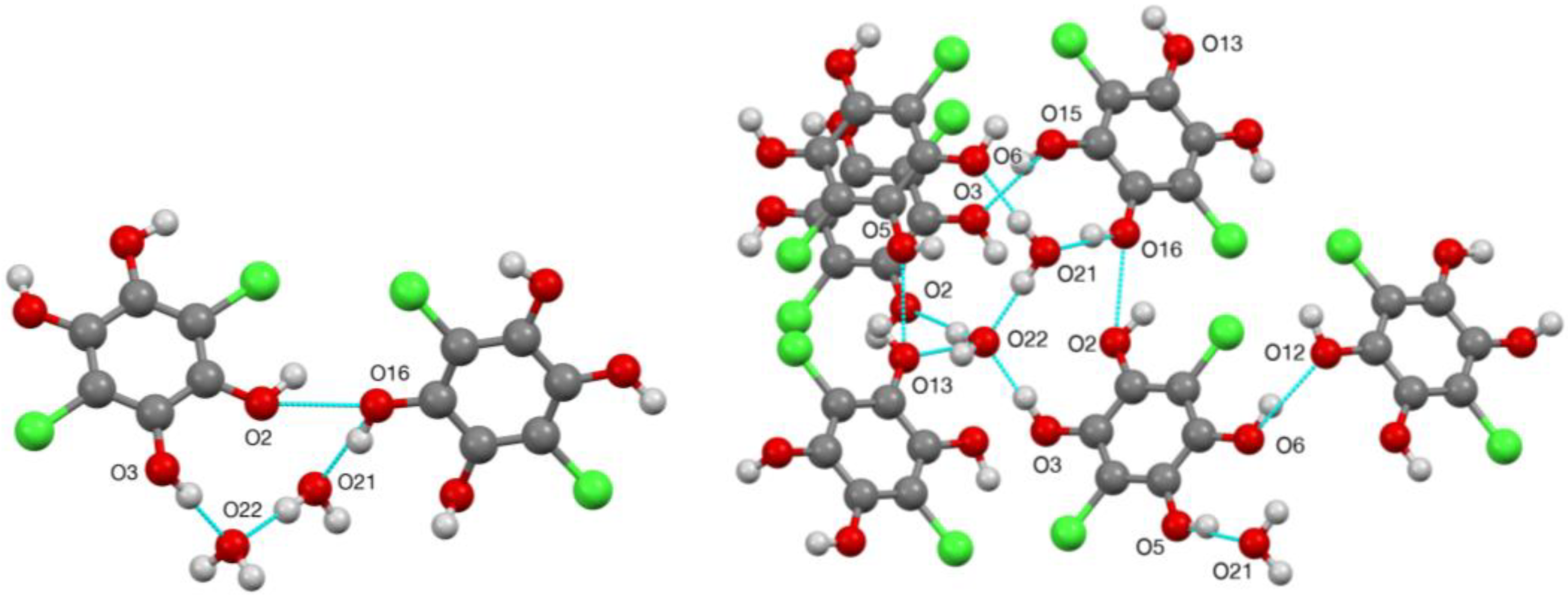
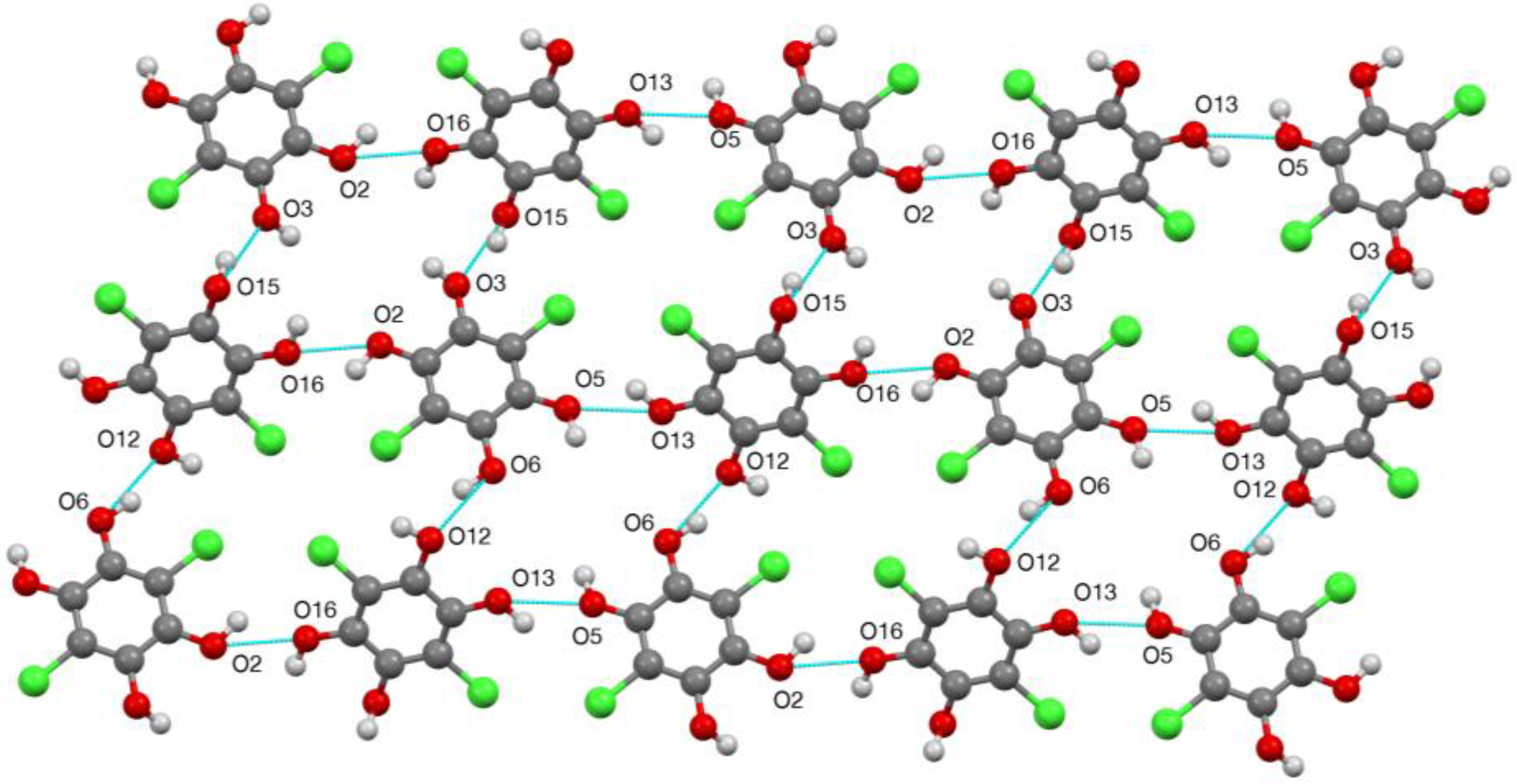
| D—H…A | D—H | H…A | D…A | D—H…A |
|---|---|---|---|---|
| O(2)–H(2)…O(16) | 0.977(16) | 2.02(3) | 2.675(2) | 122(3) |
| O(3)–H(3)…O(22) | 0.98(2) | 1.71(2) | 2.684(2) | 176(3) |
| O(5)–H(5)…O(21) | 0.98(2) | 1.73(2) | 2.695(2) | 166(3) |
| O(6)–H(6)…O(12) | 0.977(14) | 2.027(19) | 2.886(2) | 146(2) |
| O(13)–H(13)…O(5) | 0.98(2) | 2.01(4) | 2.697(2) | 126(3) |
| O(15)–H(15)…O(3) | 0.98(2) | 1.86(2) | 2.787(2) | 158(3) |
| O(16)–H(16)…O(21) | 0.98(3) | 1.70(3) | 2.659(3) | 165(3) |
| O(21)–H(21A)…O(22) | 0.979(18) | 1.757(18) | 2.734(2) | 175(2) |
| O(21)–H(21B)…O(6) | 0.98(2) | 1.89(3) | 2.868(2) | 174(2) |
| O(22)–H(22A)…O(13) | 0.98(2) | 1.83(3) | 2.772(3) | 160(3) |
| O(22)–H(22B)…O(2) | 0.98(2) | 1.83(2) | 2.799(2) | 171(3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Schindler, N.; Slawin, A.M.Z. 3,6-Dichlorobenzene-1,2,4,5-tetraol. Molbank 2022, 2022, M1415. https://doi.org/10.3390/M1415
Aitken RA, Schindler N, Slawin AMZ. 3,6-Dichlorobenzene-1,2,4,5-tetraol. Molbank. 2022; 2022(3):M1415. https://doi.org/10.3390/M1415
Chicago/Turabian StyleAitken, R. Alan, Niti Schindler, and Alexandra M. Z. Slawin. 2022. "3,6-Dichlorobenzene-1,2,4,5-tetraol" Molbank 2022, no. 3: M1415. https://doi.org/10.3390/M1415
APA StyleAitken, R. A., Schindler, N., & Slawin, A. M. Z. (2022). 3,6-Dichlorobenzene-1,2,4,5-tetraol. Molbank, 2022(3), M1415. https://doi.org/10.3390/M1415







