Abstract
Conjugates of 3H-1,2-dithiol-3-ones with various biologically active compounds are intensively investigated. Although many derivatives of this class have been described in the literature, the compounds containing two dithiole cycles have been explored much less. In this communication, it was shown that the reaction of 4,5-dichloro-3H-1,2-dithiol-3-one with piperazine can selectively lead to the mono-product, 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one and bis-product, 5,5′-(piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one). The structure of the synthesized compounds was established by elemental analysis, high resolution mass-spectrometry, 1H, 13C NMR and IR spectroscopy, and mass-spectrometry.
1. Introduction
Among 1,2-dithioles, 3H-1,2-dithiole-3-thiones have been widely studied [1,2,3] as compounds that endogenously produce hydrogen sulfide (H2S), the third gaseous signaling molecule [4]. It is known that 1,2-dithiole-3-thiones can extrude sulfur and convert to 1,2-dithiol-3-ones, which are also characterized by many types of biological actions, particularly cancer prevention [5,6,7], amongst other activities [8,9]. In some cases, keto-analogues showed an activity comparable to the effects of the corresponding thione derivatives. Thus, the keto-analogue of the active ingredient of the cancer-preventive drug oltipraz, 4-methyl-5-(pyrazin-2-yl)-3H-1,2-dithiol-3-one (Figure 1), increased the activity of the detoxifying cytoprotective enzyme DT-diaphorase in HT29 intestinal adenocarcinoma cells by 2.8 times compared to the control, while this factor for oltipraz itself was only 2.6 times [10].
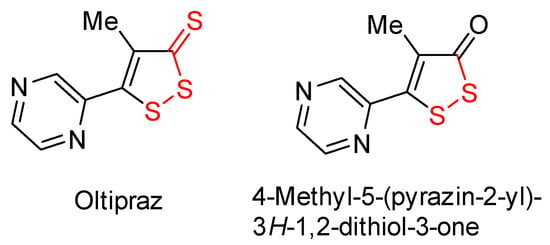
Figure 1.
Oltipraz and 4-methyl-5-(pyrazin-2-yl)-3H-1,2-dithiol-3-one.
Among dithiolones, the readily available 4,5-dichloro-3H-1,2-dithiol-3-one 1 [11] can be considered an attractive starting material for many biologically active molecules due to the easy reactivity of the 5-chlorine atom in 5-chloro-3H-1,2-dithiol-3-ones [12,13,14]. Piperazine is a remarkable scaffold in the generation of diverse pharmacological agents [15]. We have suggested that the combination of the piperazine scaffold together with one or with two 3H-1,2-dithiol-3-one cores can provide novel biologically important compounds. Herein, we report the reaction of 4,5-dichloro-3H-1,2-dithiol-3-one 1 with piperazine with the selective formation of 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2 and 5,5′-(piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one) 3.
2. Results and Discussion
The treatment of 4,5-dichloro-3H-1,2-dithiol-3-one 1 with an excess of piperazine in methanol at room temperature led to a mono-product 2 with a high yield (Scheme 1, Table 1, Entry 1). At the same time, carrying out the reaction at a higher temperature with a slightly smaller (1.5 eqv) excess of piperazine gave a mixture of 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2 and 5,5′-(piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one) 3 (Entry 2). For the selective formation of bis-product 3, we decided to use a base (triethylamine) to bind the released hydrogen chloride. However, conducting the reaction in methanol at room temperature also resulted in a mixture of mono- 2 and bis-product 3 (Entry 3). The refluxing of a similar reaction mixture led to the selective formation of bis-product 3 with a high yield (Entry 4). The use of acetonitrile as a solvent instead of methanol, although it reduced the reaction time, did not increase the yield of the final product 3 (Entry 5). A separate experiment has shown that the treatment of mono-product 2 with dichlorodithiol 1 in the presence of triethylamine led to bis-product 3.
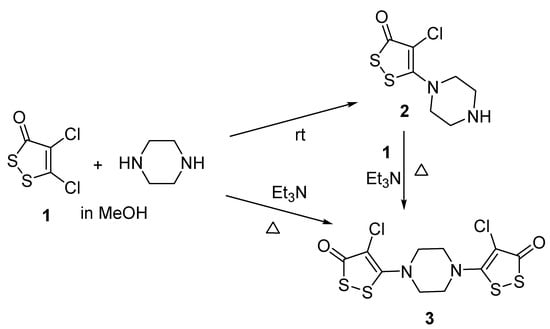
Scheme 1.
Synthesis of 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2 and 5,5′-(piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one) 3.

Table 1.
Reaction of 4,5-dichloro-3H-1,2-dithiol-3-one 1 with piperazine.
The structure of 5,5′-(piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one) 3 and 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2 was fully confirmed by elemental analysis, high resolution mass-spectrometry, 1H, 13C NMR and IR spectroscopy, and mass-spectrometry.
In conclusion, the selective synthesis of 5-piperazinyl derivatives of 1,2-dithiol-3-one 2 and 3 was developed by the reaction of 4,5-dichloro-3H-1,2-dithiol-3-one 1 with piperazine. The compounds obtained may have useful pharmacological properties.
3. Materials and Methods
4,5-Dichloro-1,2-dithiol-3-one 1 was prepared according to the published method [12]. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin ElmerInc., Waltham, MA, USA). The melting point was determined on a Kofler hot-stage apparatus and is uncorrected. The 1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300 and 75 MHz) with TMS as the standard. The MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). The IR spectrum was measured with a Bruker “Alpha-T” instrument in a KBr pellet. The high-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI).
Synthesis of 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2 (Supplementary Materials, Figures S1–S5).
A solution of 4,5-dichloro-1,2-dithiol-3-one 1 (94 mg, 0.5 mmol) in MeOH (5 mL) was added to a solution of piperazine (86 mg, 1 mmol) in MeOH (10 mL). The reaction mixture was stirred for 30 min at room temperature. The solvent was distilled, and the residue was treated with water (10 mL) and extracted with CH2Cl2 (2 × 20 mL). Combined organic extracts were washed with brine and dried with MgSO4. The solvent was removed, and the residue was treated with water, filtered, washed with water (5 mL) and dried. Yield 98 mg (83%), yellow solid, mp. 90–92 °C. IR spectrum (KBr), ν, cm−1: 3448 (C-H), 3342 (N-H), 2956 and 2824 (C-H), 1631 (C=O), 1532, 1442 (CH2), 1327, 1258, 1198, 1027 (C-Cl), 814, 778, 648, 517. 1H-NMR (DMSO-d6, ppm): δ 2.86 (4H, m), 3.61 (4H, m). 13C-NMR (DMSO-d6, ppm): 45.4 (2 C-H), 51.1 (2 C-H), 98.5 (C-Cl), 168.7 (C-N), 183.2 (C=O). MS (EI, 70 Ev), m/z (I, %): 238 (M + 2, 15), 236 (M+, 45), 201 (100), 194 (20), 172 (10), 137 (25), 91 (15), 87 (20), 64 (45), 56 (82), 42 (30). HRMS (ESI-TOF): calcd for C7H10ClN2OS2 [M + H]+ 236.9921; found m/z 236.9918; calcd for C7H9ClN2NaOS2 [M + Na]+ 258.9741, found m/z 258.9737.
Synthesis of 5,5′-(piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one) 3 (Supplementary Materials, Figures S6–S10).
- From 4,5-dichloro-1,2-dithiol-3-one 1
Triethylamine (0.28 mL, 2 mmol) and piperazine (43 mg, 0.5 mmol) were added to a solution of 4,5-dichloro-1,2-dithiol-3-one 1 (187 mg, 1 mmol) in MeOH (15 mL), and the reaction mixture was refluxed for 2 h. The residue was treated with water (10 mL), filtered, washed with 2M solution HCl (3 mL), brine (3 mL) and dried. Yield 172 mg (89%), dark yellow solid, mp. 78–80 °C. IR spectrum (KBr), ν, cm−1: 3467, 2925 and 2852 (all C-H), 1648 (C=O), 1528, 1438 (CH2), 1376, 1281, 1197, 1006 (C-Cl), 822, 775, 477. 1H-NMR (DMSO-d6, ppm): δ 3.91 (8H, br s). 13C-NMR (DMSO-d6, ppm): 48.6 (4 C-H), 98.8 (C-Cl), 167.9 (C-N), 182.8 (C=O). MS (EI, 70 Ev), m/z (I, %): 388 (M + 2, 10), 386 (M+, 15), 351 (5), 256 (10), 201 (10), 128 (15), 96 (15), 64 (100), 32 (45). HRMS (ESI-TOF): calcd for C10H9Cl2N2O2S4 [M + H]+ 386.8920; found m/z 386.8918; calcd for C10H8Cl2N2NaO2S4 [M + Na]+ 408.8737, found m/z 408.8738.
- b.
- From 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2
Triethylamine (0.28 mL, 2 mmol) was added to a solution of 4,5-dichloro-1,2-dithiol-3-one 1 (187 mg, 1 mmol) and 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2 (118 mg, 1 mmol) in MeOH (15 mL), and the reaction mixture was refluxed for 2 h. The residue was treated with water (10 mL), filtered, washed with 2M solution HCl (3 mL), brine (3 mL) and dried. Yield 328 mg (85%).
Supplementary Materials
The following are available online: copies of 1H, 13C NMR, IR, HRMS and mass-spectra for the compounds 2 (Figures S1–S5) and 3 (Figures S6–S10).
Author Contributions
Conceptualization, V.A.O.; methodology, O.A.R.; software, V.A.O.; validation, O.A.R.; formal analysis, investigation, V.A.O.; resources, O.A.R.; data curation, O.A.R.; writing—original draft preparation, V.A.O.; writing—review and editing, V.A.O.; visualization, O.A.R.; supervision, O.A.R.; project administration, O.A.R.; funding acquisition, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rakitin, O.A. Synthesis and reactivity of 3H-1,2-dithiole-3-thiones. Molecules 2021, 26, 3595. [Google Scholar] [CrossRef] [PubMed]
- Marković, R.; Rašović, A. 1,2-Dithioles. In Comprehensive Heterocyclic Chemistry III; Joule, J.A., Ed.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 893–954. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and studies of acetylthioglycoside conjugates of 4-chloro-1,2-dithiole-3-thione as potential antitumor agents. Russ. Chem. Bull. 2021, 70, 573–579. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelloff, G.J.; Boone, C.W.; Crowell, J.A.; Steele, V.E.; Lubet, R.; Sigman, C.C. Chemopreventive drug development: Perspectives and progress. Cancer Epidemiol. Biomark. Prev. 1994, 3, 85–98. [Google Scholar]
- Kensler, T.W.; He, X.; Otieno, M.; Egner, P.A.; Jacobson, L.P.; Chen, B.; Wang, J.S.; Zhu, Y.R.; Zhang, B.C.; Wang, J.B.; et al. Oltipraz chemoprevention trial in Qidong, People’s Republic of China: Modulation of serum aflatoxin albumin adduct biomarkers. Cancer Epidemiol. Biomark. Prev. 1998, 7, 127–134. [Google Scholar]
- Prochaska, H.J.; DeLong, M.J.; Talalay, P. On the mechanisms of induction of cancer-protective enzymes: A unifying proposal. Proc. Natl. Acad. Sci. USA 1985, 82, 8232–8236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Reeve, A.M.; Desai, U.R.; Kellogg, G.E.; Reynolds, K.A. 1,2-Dithiole-3-Ones as Potent Inhibitors of the Bacterial 3-Ketoacyl Acyl Carrier Protein Synthase III (FabH). Antimicrob. Agents Chemother. 2004, 48, 3093–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 1,2-Dithiolone Compounds and Use Thereof. WO Patent WO2020/35826, 20 February 2020.
- O’Dwyer, P.J.; Clayton, M.; Halbherr, T.; Myers, C.B.; Yao, K.-S. Cellular kinetics of induction by oltipraz and its keto derivative of detoxication enzymes in human colon adenocarcinoma cells. Clin. Cancer Res. 1997, 3, 783–791. [Google Scholar]
- Boberg, F. Über 1.2-Dithia-cyclopentene, V. 4.5-Dichlor-1.2-dithia-cyclopentenon-(3). Liebigs Ann. Chem. 1964, 679, 109–118. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Agafonova, I.G.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and study of thioglycoside conjugates of 4-chloro-1,2-dithiol-3-one as potential cancer-preventive substances in vitro and in vivo. Russ Chem Bull. 2022, 71, 489–495. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Berezin, A.A.; Lysov, K.A.; Rakitin, O.A. Selective synthesis of bis[1,2]dithiolo[1,4]thiazines from 4-isopropylamino-5-chloro-1,2-dithiole-3-ones. Tetrahedron Lett. 2007, 48, 5851–5854. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Rakitin, O.A. Reactivity of 1,2-dithioles. Russ. Chem. Revi. 2012, 81, 638–661. [Google Scholar] [CrossRef]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).