5,5′-(Piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
- From 4,5-dichloro-1,2-dithiol-3-one 1
- b.
- From 4-chloro-5-piperazin-1-yl-3H-1,2-dithiol-3-one 2
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rakitin, O.A. Synthesis and reactivity of 3H-1,2-dithiole-3-thiones. Molecules 2021, 26, 3595. [Google Scholar] [CrossRef] [PubMed]
- Marković, R.; Rašović, A. 1,2-Dithioles. In Comprehensive Heterocyclic Chemistry III; Joule, J.A., Ed.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 893–954. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and studies of acetylthioglycoside conjugates of 4-chloro-1,2-dithiole-3-thione as potential antitumor agents. Russ. Chem. Bull. 2021, 70, 573–579. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelloff, G.J.; Boone, C.W.; Crowell, J.A.; Steele, V.E.; Lubet, R.; Sigman, C.C. Chemopreventive drug development: Perspectives and progress. Cancer Epidemiol. Biomark. Prev. 1994, 3, 85–98. [Google Scholar]
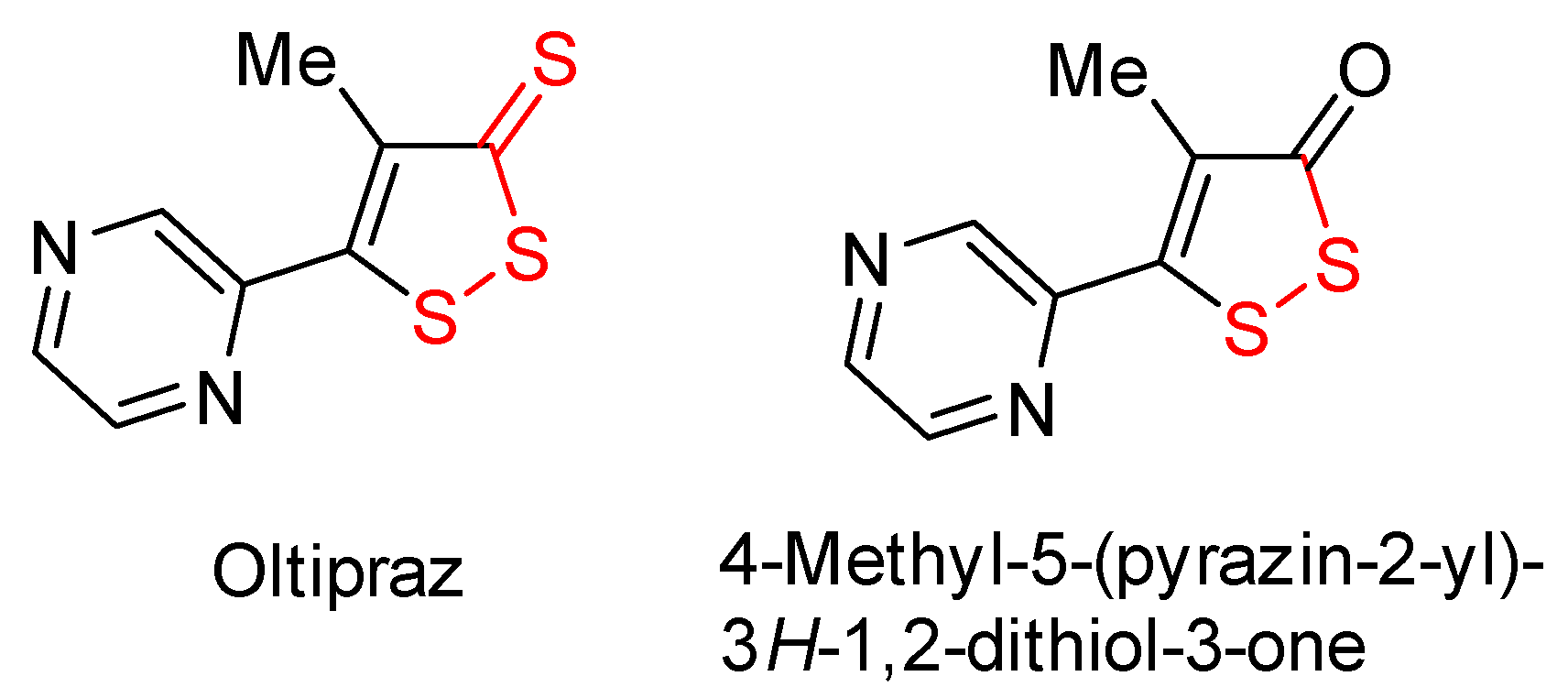
- Kensler, T.W.; He, X.; Otieno, M.; Egner, P.A.; Jacobson, L.P.; Chen, B.; Wang, J.S.; Zhu, Y.R.; Zhang, B.C.; Wang, J.B.; et al. Oltipraz chemoprevention trial in Qidong, People’s Republic of China: Modulation of serum aflatoxin albumin adduct biomarkers. Cancer Epidemiol. Biomark. Prev. 1998, 7, 127–134. [Google Scholar]
- Prochaska, H.J.; DeLong, M.J.; Talalay, P. On the mechanisms of induction of cancer-protective enzymes: A unifying proposal. Proc. Natl. Acad. Sci. USA 1985, 82, 8232–8236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Reeve, A.M.; Desai, U.R.; Kellogg, G.E.; Reynolds, K.A. 1,2-Dithiole-3-Ones as Potent Inhibitors of the Bacterial 3-Ketoacyl Acyl Carrier Protein Synthase III (FabH). Antimicrob. Agents Chemother. 2004, 48, 3093–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 1,2-Dithiolone Compounds and Use Thereof. WO Patent WO2020/35826, 20 February 2020.
- O’Dwyer, P.J.; Clayton, M.; Halbherr, T.; Myers, C.B.; Yao, K.-S. Cellular kinetics of induction by oltipraz and its keto derivative of detoxication enzymes in human colon adenocarcinoma cells. Clin. Cancer Res. 1997, 3, 783–791. [Google Scholar]
- Boberg, F. Über 1.2-Dithia-cyclopentene, V. 4.5-Dichlor-1.2-dithia-cyclopentenon-(3). Liebigs Ann. Chem. 1964, 679, 109–118. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Agafonova, I.G.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and study of thioglycoside conjugates of 4-chloro-1,2-dithiol-3-one as potential cancer-preventive substances in vitro and in vivo. Russ Chem Bull. 2022, 71, 489–495. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Berezin, A.A.; Lysov, K.A.; Rakitin, O.A. Selective synthesis of bis[1,2]dithiolo[1,4]thiazines from 4-isopropylamino-5-chloro-1,2-dithiole-3-ones. Tetrahedron Lett. 2007, 48, 5851–5854. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Rakitin, O.A. Reactivity of 1,2-dithioles. Russ. Chem. Revi. 2012, 81, 638–661. [Google Scholar] [CrossRef]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]

| Entry | Solvent | Piperazine (equiv) | Et3N (equiv) | Temperature, °C | Time, h | Yield, % | |
|---|---|---|---|---|---|---|---|
| 2 | 3 | ||||||
| 1 | MeOH | 2 | 0 | 25 | 0.5 | 85 | 0 |
| 2 | MeOH | 1.5 | 0 | 65 | 1 | 71 | 15 |
| 3 | MeOH | 0.5 | 2 | 25 | 6 | 33 | 51 |
| 4 | MeOH | 0.5 | 2 | 65 | 2 | 0 | 89 |
| 5 | MeCN | 0.5 | 2 | 81 | 1 | 0 | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogurtsov, V.A.; Rakitin, O.A. 5,5′-(Piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one). Molbank 2022, 2022, M1411. https://doi.org/10.3390/M1411
Ogurtsov VA, Rakitin OA. 5,5′-(Piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one). Molbank. 2022; 2022(3):M1411. https://doi.org/10.3390/M1411
Chicago/Turabian StyleOgurtsov, Vladimir A., and Oleg A. Rakitin. 2022. "5,5′-(Piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one)" Molbank 2022, no. 3: M1411. https://doi.org/10.3390/M1411
APA StyleOgurtsov, V. A., & Rakitin, O. A. (2022). 5,5′-(Piperazine-1,4-diyl)bis(4-chloro-3H-1,2-dithiol-3-one). Molbank, 2022(3), M1411. https://doi.org/10.3390/M1411







