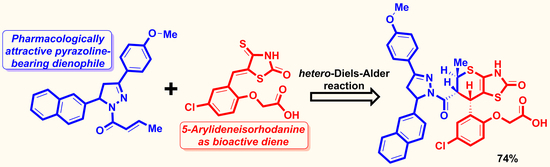
rel-2-[4-Chloro-2-[(5R,6R,7S)-6-[5-(4-methoxyphenyl)-3-(2-naphthyl)-3,4-dihydropyrazole-2-carbonyl]-5-methyl-2-oxo-3,5,6,7-tetrahydrothiopyrano[2,3-d]thiazol-7-yl]phenoxy]acetic Acid
Abstract
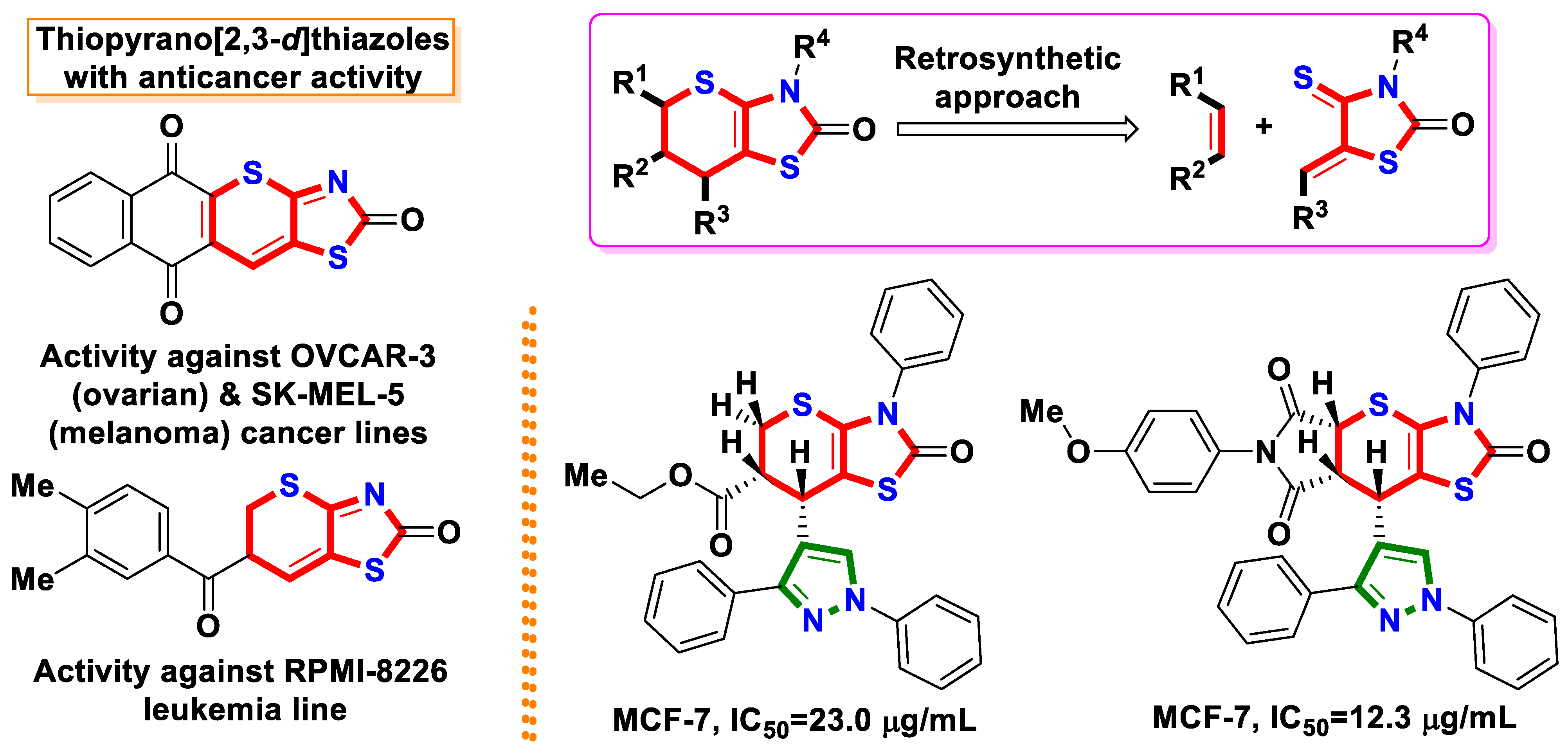
:1. Introduction
2. Results and Discussion
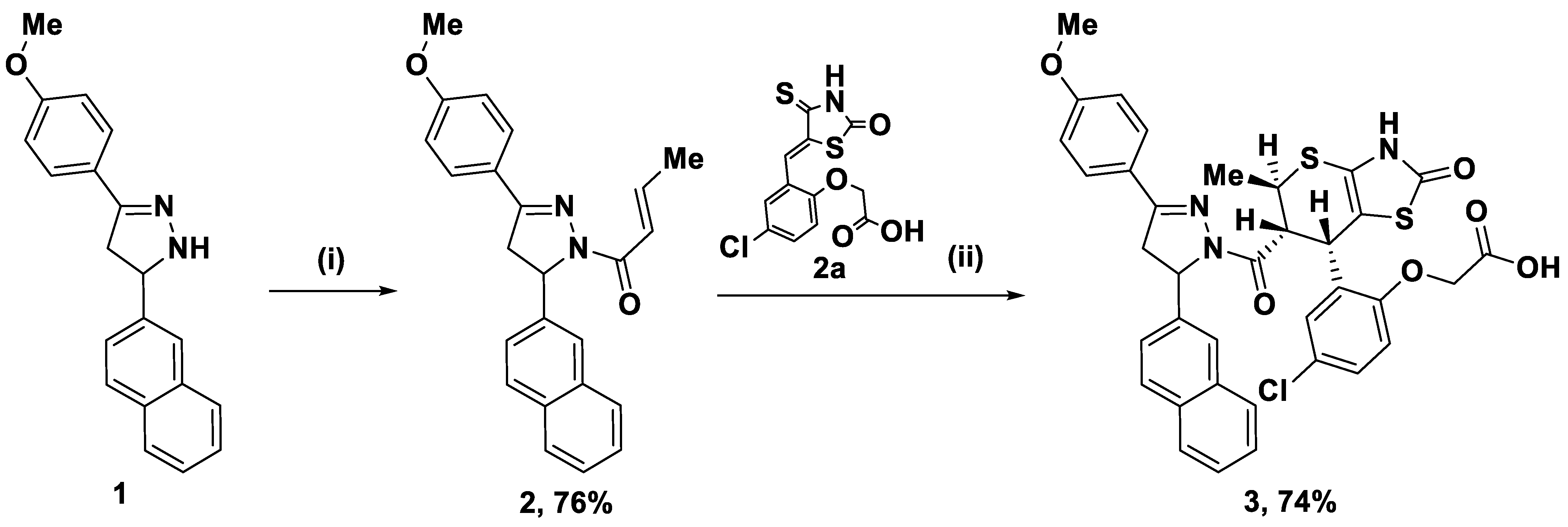
2.1. Synthesis of the Title Compound 3
2.2. In Vitro Evaluation of the Anticancer Activity of Compound 3
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atamanyuk, D.; Zimenkovsky, B.; Atamanyuk, V.; Lesyk, R. 5-Ethoxymethylidene-4-thioxo-2-thiazolidinone as versatile building block for novel biorelevant small molecules with thiopyrano[2,3-d][1,3]thiazole core. Synth. Commun. 2014, 44, 237–244. [Google Scholar] [CrossRef]
- Metwally, N.H.; Badawy, M.A.; Okpy, D.S. Green synthesis of some new thiopyrano[2,3-d][1,3]thiazoles using lemon juice and their antibacterial activity. Synth. Commun. 2018, 48, 2496–2509. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Golota, S.; Zimenkovsky, B.; Atamanyuk, D.; Gzella, A.; Lesyk, R. Synthesis, anticancer and antiviral activities of novel thiopyrano[2,3-d]thiazole-6-carbaldehydes. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1245–1249. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Wu, S.; Zhu, S.; Dong, G.; Miao, Z.; Yao, J.; Zhang, W.; Sheng, C.; Wang, W. Facile construction of structurally diverse thiazolidinedione-derived compounds via divergent stereoselective cascade organocatalysis and their biological exploratory studies. ACS Comb. Sci. 2013, 15, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017-Utility and Limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhong, H.; Wang, K.; Li, N.; Chen, L. Gains from no real PAINS: Where ‘Fair Trial Strategy’ stands in the development of multi-target ligands. Acta Pharm. Sin. B 2021, 11, 3417–3432. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Holota, S.; Yushyn, I.; Sabadakh, O.; Karpenko, O.; Novikov, V.; Lesyk, R. Synthesis and Biological Activity Evaluation of Polyfunctionalized Anthraquinonehydrazones. Lett. Drug Des. Discov. 2021, 18, 199–209. [Google Scholar] [CrossRef]
- Metwally, N.H.; Badawy, M.A.; Okpy, D.S. Synthesis and anticancer activity of some new thiopyrano[2,3-d]thiazoles incorporating pyrazole moiety. Chem. Pharm. Bull. 2015, 63, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Qiu, K.M.; Cui, H.E.; Yang, Y.S.; Xing, M.; Qiu, X.Y.; Bai, L.F.; Zhu, H.L. Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives containing benzodioxole as potential anticancer agents. Bioorg. Med. Chem. 2013, 21, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.; Sahoo, C.R.; Nandini Sarangi, P.K.; Prusty, S.K.; Padhy, R.N.; Paidesetty, S.K. Molecules with versatile biological activities bearing antipyrinyl nucleus as pharmacophore. Eur. J. Med. Chem. 2020, 186, 111911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; et al. Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect. Eur. J. Med. Chem. 2020, 186, 111893. [Google Scholar] [CrossRef] [PubMed]
- Holota, S.; Yushyn, I.; Khyluk, D.; Vynnytska, R.; Lesyk, R. N-(3-Cyano-4,5,6,7-tetrahydrobenzothio- phen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide. Molbank 2021, 2021, M1211. [Google Scholar] [CrossRef]
- Yushyn, I.; Holota, S.; Lesyk, R. 2,2-Dichloro-N-[5-[2-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]-2-oxoethyl] sulfanyl-1,3,4-thiadiazol-2-yl]acetamide. Molbank 2022, 2022, M1328. [Google Scholar] [CrossRef]
- Palaska, E.; Aytemir, M.; Uzbay, I.T.; Erol, D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001, 36, 539–543. [Google Scholar] [CrossRef]
- Holota, S.; Lozynskyi, A.; Konechnyi, Y.; Shepeta, Y.; Lesyk, R. 5-[4-(tert-Butyl)cyclohexylidene]-2-thioxothiazolidin-4-one. Molbank 2021, 2021, M1281. [Google Scholar] [CrossRef]
- Zelisko, N.; Atamanyuk, D.; Vasylenko, O.; Bryhas, A.; Matiychuk, V.; Gzella, A.; Lesyk, R. Crotonic, cynnamic, and propiolic acids motifs in the synthesis of thiopyrano[2,3-d][1,3]thiazoles via hetero-Diels–Alder reaction and related tandem processes. Tetrahedron 2014, 70, 720–729. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI In Vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 2, pp. 23–42. [Google Scholar]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]

| Compound | 60 Cell Lines Assay in One Dose, 10 μM | |
|---|---|---|
| Mean Growth, % | Most Sensitive Cell Line(s) Growth Inhibition Percent/Line/Panel | |
| 3 | 104.68 | 92.48/RPMI-8226/Leukemia 92.77/CCRF-CEM/Leukemia 92.90/K-562/Leukemia 92.74/SF-539/CNS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yushyn, I.; Holota, S.; Ivantsiv, O.; Lesyk, R. rel-2-[4-Chloro-2-[(5R,6R,7S)-6-[5-(4-methoxyphenyl)-3-(2-naphthyl)-3,4-dihydropyrazole-2-carbonyl]-5-methyl-2-oxo-3,5,6,7-tetrahydrothiopyrano[2,3-d]thiazol-7-yl]phenoxy]acetic Acid. Molbank 2022, 2022, M1410. https://doi.org/10.3390/M1410
Yushyn I, Holota S, Ivantsiv O, Lesyk R. rel-2-[4-Chloro-2-[(5R,6R,7S)-6-[5-(4-methoxyphenyl)-3-(2-naphthyl)-3,4-dihydropyrazole-2-carbonyl]-5-methyl-2-oxo-3,5,6,7-tetrahydrothiopyrano[2,3-d]thiazol-7-yl]phenoxy]acetic Acid. Molbank. 2022; 2022(3):M1410. https://doi.org/10.3390/M1410
Chicago/Turabian StyleYushyn, Ihor, Serhii Holota, Oksana Ivantsiv, and Roman Lesyk. 2022. "rel-2-[4-Chloro-2-[(5R,6R,7S)-6-[5-(4-methoxyphenyl)-3-(2-naphthyl)-3,4-dihydropyrazole-2-carbonyl]-5-methyl-2-oxo-3,5,6,7-tetrahydrothiopyrano[2,3-d]thiazol-7-yl]phenoxy]acetic Acid" Molbank 2022, no. 3: M1410. https://doi.org/10.3390/M1410
APA StyleYushyn, I., Holota, S., Ivantsiv, O., & Lesyk, R. (2022). rel-2-[4-Chloro-2-[(5R,6R,7S)-6-[5-(4-methoxyphenyl)-3-(2-naphthyl)-3,4-dihydropyrazole-2-carbonyl]-5-methyl-2-oxo-3,5,6,7-tetrahydrothiopyrano[2,3-d]thiazol-7-yl]phenoxy]acetic Acid. Molbank, 2022(3), M1410. https://doi.org/10.3390/M1410