Abstract
A new synthetic procedure for obtaining two previously reported donor-acceptor butadiene dyes, namely 5-(3,3-bis(4-methoxyphenyl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione and 5-(3,3-bis(4-(dimethylamino)phenyl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione, based on the InCl3-catalyzed coupling 1,3-diethyl-2-thiobarbituric acid with 1,1-bis(4-methoxyphenyl)prop-2-yn-1-ol and 1,1-bis(4-(dimethylamino)phenyl)prop-2-yn-1-ol, respectively, is presented. The reactions, which cleanly proceed in water under MW irradiation, involve the initial generation of the corresponding enals by Meyer-Schuster rearrangement of the alkynols and their subsequent Knoevenagel condensation with the 2-thiobarbituric acid derivative. By following the same approach, the novel butadiene 5-(3,3-bi([1,1′-biphenyl]-4-yl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione, which was characterized by 1H and 13C{1H} NMR, IR, UV-Vis, elemental analysis and HRMS, was synthesized in 79% yield.
1. Introduction
Push–pull chromophores, in which electron donor and electron acceptor groups are connected through a π-conjugated system (Figure 1), are the subject of considerable research interest in material science due to their unique properties (color, electrochemical, photochemical and solvatochromic behavior, nonlinear optical (NLO) properties, etc.) [1,2,3,4,5].

Figure 1.
Simplified representation of a push–pull molecule.
A wide variety of donor and acceptor units, as well as π-conjugated spacers, have been combined during the last decades for the construction of push–pull molecules. In this context, the pseudoaromatic 2-thioxodihydropyrimidine-4,6(1H,5H)-dione ring of thiobarbituric acid and its N-alkylated derivatives has been extensively employed as an electron-withdrawing moiety in push–pull chromophores [6,7,8,9]. Representative examples are dyes 3a,b, recently described by Dumur and co-workers, featuring p-methoxyphenyl and p-dimethylaminophenyl donor groups connected to the barbituric acid skeleton through a 1,3-butadiene chain spacer (Scheme 1), which showed a marked solvatochromic behavior in solution [10,11]. As shown in Scheme 1, compounds 3a,b were obtained by Knoevenagel condensation of commercially available 1,3-diethyl-2-thiobarbituric acid 1 with the corresponding α,β-unsaturated aldehyde 2a,b in refluxing ethanol under basic conditions. For the preparation of enals 2a,b, Dumur and co-workers followed a classical synthetic route involving the initial olefination of the respective 4,4′-disubstituted benzophenone, and subsequent Vilsmeier formylation of the resulting 1,1-diarylethylenes (see Scheme 1).
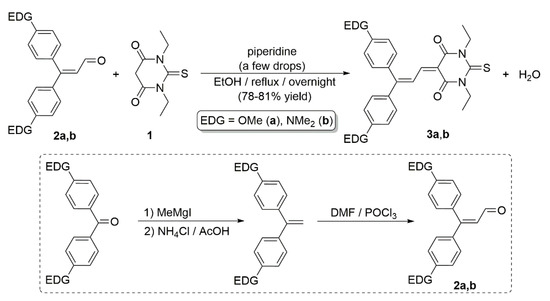
Scheme 1.
Synthesis of dyes 3a,b reported by Dumur and co-workers.
It is well known that α,β-unsaturated carbonyl compounds (both enals and enones) can be accessed, in a straightforward and atom-economical manner, through the catalytic Meyer–Schuster rearrangement of propargylic alcohols [12,13,14]. In this context, we developed some years ago an efficient, general and environmentally benign protocol for the Meyer–Schuster conversion of terminal propargylic alcohols into enals employing inexpensive InCl3 as the catalyst, water as solvent, and microwaves (MW) irradiation as the heating source (Scheme 2) [15].
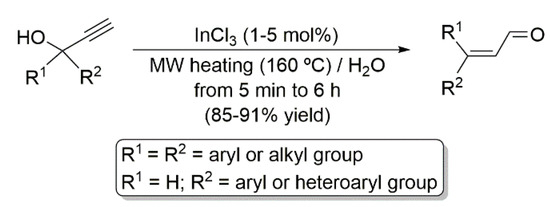
Scheme 2.
MW-assisted InCl3-catalyzed Meyer–Schuster rearrangement of terminal propargylic alcohols in water.
Additionally, in line with the interest of our group in the design of push–pull molecules [16,17], we demonstrated the utility of this MW-assisted InCl3-catalyzed reaction in the field with the high-yield synthesis of the donor-acceptor butadienes 6a,b, structurally related to 3a,b, by coupling of the corresponding propargylic alcohols 4a,b with indan-1,3-dione 5 [18] As shown in Scheme 3, under the reaction conditions employed, the Meyer–Schuster and Knoevenagel reactions run smoothly and successively in a one-pot manner. Taking advance of this previous work, herein we would like to communicate that Dumur’s dyes 3a,b can also be generated by direct MW-assisted InCl3-catalyzed coupling 1,3-diethyl-2-thiobarbituric acid 1 with alkynols 4a,b. In addition, the synthesis and spectroscopic characterization of the novel butadiene 5-(3,3-bi([1,1’-biphenyl]-4-yl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 3c will be presented.
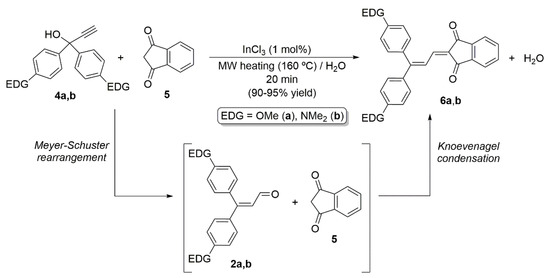
Scheme 3.
MW-assisted InCl3-catalyzed synthesis of the push–pull butadienes 6a,b.
2. Results and Discussion
As shown in Scheme 4, we found that by applying the same reaction conditions previously employed in the preparation of the indan-1,3-dione-based dyes 6a,b, the push–pull butadienes 3a,b can be accessed in 75 and 65% yield, respectively. A notable aspect of this new synthetic route is the ease reaction work-up procedure to isolate the products since, after the MW-irradiation period (20 min at 160 °C) and cooling of the mixture to room temperature, both derivatives appear as precipitated solids. In this way, a simple decantation and consecutive washing with water, methanol and diethyl ether delivered 3a,b in pure form. The recorded IR, NMR (1H and 13C{1H}) and HRMS spectra were in full agreement with the characterization data reported by Dumur and co-workers (full details are given in the Materials and Methods section and copies of the spectra included in the Supplementary Materials) [10,11].
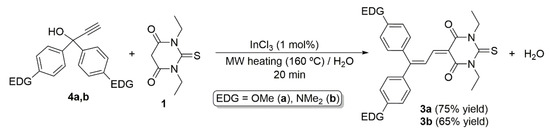
Scheme 4.
MW-assisted InCl3-catalyzed synthesis of butadienes 3a,b from 1,3-diethyl-2-thiobarbituric acid 1 and propargylic alcohols 4a,b.
The synthetic utility of this MW-assisted InCl3-catalyzed process was further demonstrated with the preparation of the novel butadiene 5-(3,3-bi([1,1’-biphenyl]-4-yl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 3c starting from stoichiometric amounts of 1,3-diethyl-2-thiobarbituric acid 1 and 1,1-di([1,1’-biphenyl]-4-yl)prop-2-yn-1-ol 4c (Scheme 5). Compound 3c was isolated as a red solid in 79% yield and characterized by 1H and 13C{1H] NMR, IR, elemental analysis and HRMS, with all data being fully consistent with the proposed formulation (full details are given in the Materials and Methods section and copies of the spectra included in the Supplementary Materials). Thus, its IR spectrum showed characteristic C=O vibrations appearing as a strong and broad absorption band at 1667 cm−1. The generation of a butadiene >C=CH-CH=C< unit was clearly reflected in the 1H NMR spectrum by the appearance of two doublet signals at 8.30 and 8.73 ppm (3JHH = 12.3 Hz), the spectrum also showing the typical signals for the two inequivalent ethyl groups of the N-alkylated thiobarbituric acid skeleton, i.e., two quartets at δH 4.54 and 4.62 ppm and two triplets at δH 1.31 and 1.38 ppm for the CH2 and CH3 units, respectively (3JHH = 6.9 Hz). The 13C{1H] NMR spectrum of 3c also showed the expected signals, with the most quickly identifiable being that of the C=S unit, which appears as the most deshielded one in the spectrum (δC 178.9 ppm).
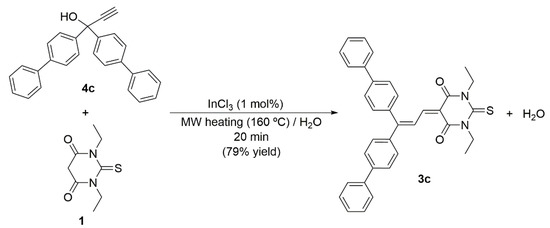
Scheme 5.
MW-assisted InCl3-catalyzed synthesis of the novel butadiene 3c.
Finally, the optical properties of the novel butadiene 3c were briefly investigated by means of UV-Vis spectroscopy in heptane and methyl sulfoxide (DMSO) solution (see Table 1; copies of the spectra are included in the Supplementary Materials file). As expected, the spectra displayed an intense absorption band in the visible region, hypsochromically shifted when compared to that of compounds 3a,b due to the lower electron-donor capability of the biphenyl units vs. the p-methoxyphenyl and p-dimethylaminophenyl ones. In addition, similarly to the case of 3a,b, 3c also features a positive solvatochromic behavior, its absorption maximum undergoing a red shift on going from the non-polar heptane solvent to the highly polar DMSO one (Δλmax = 29 nm).

Table 1.
λmax values of butadienes 3a–c in heptane and dimethyl sulfoxide solution.
3. Materials and Methods
Indium(III) chloride and 1,3-diethyl-2-thiobarbituric acid 1 were obtained from Merck KGaA (Darmstadt, Germany) and used as received. The propargylic alcohols 4a,b [19] and 4c [20] were synthesized following the methods reported in the literature. Organic solvents were dried by standard methods and distilled under argon before use [21]. NMR spectra were recorded on a Bruker DPX-300 (Billerica, MA, USA) spectrometer. The residual signal of the deuterated solvent (CDCl3) was employed as reference for the chemical shifts. The PerkinElmer 1720-XFT and Lambda 25 spectrometers (Waltham, MA, USA) were used for IR and UV-Vis measurements, respectively. HRMS data were provided by the General Services of the University of Oviedo employing a QTOF Bruker Impact II mass spectrometer. Elemental analyses were provided by the Analytical Service of the Instituto de Investigaciones Químicas (IIQ-CSIC) of Seville using a LECO TruSpec CHN analyzer (St. Joseph, MI, USA).
3.1. General Procedure for the Preparation of Butadienes 3a–c
A pressure-resistant septum-sealed glass vial was charged with 1,3-diethyl-2-thiobarbituric acid (1; 0.101 g, 0.5 mmol), the corresponding propargylic alcohol (4a–c; 0.5 mmol), InCl3 (0.001 g, 0.005 mmol), a magnetic stirrer bar and water (0.5 mL). The vial was then placed inside the cavity of a CEM Discover© S-Class microwave synthesizer (Matthews, NC, USA) and power was held at 300 W until the desired temperature was reached (160 °C). Microwave power was automatically regulated for the remainder of the experiment to maintain the temperature (monitored by a built-in infrared sensor). The internal pressure during the reaction ranged between 10 and 70 psi. After 20 min of irradiation, the vial was cooled to room temperature, the reaction mixture transferred to a flask, and the solid precipitate washed with water (1 × 5 mL), methanol (1 × 5 mL) and diethyl ether (1 × 5 mL). Characterization data for the resulting compounds 3a–c are as follows.
3.2. 5-(3,3-Bis(4-methoxyphenyl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (3a)
Red solid. Yield: 0.168 g (75%). 1H NMR (300 MHz, CDCl3): δ = 8.52 (d, 1H, JHH = 12.7 Hz), 8.20 (d, 1H, JHH = 12.7 Hz), 7.47 (d, 2H, JHH = 8.9 Hz), 7.23 (d, 2H, JHH = 8.7 Hz), 7.02 (d, 2H, JHH = 8.7 Hz), 6.92 (d, 2H, JHH = 8.9 Hz), 4.58 (q, 2H, JHH = 7.0 Hz), 4.52 (q, 2H, JHH = 7.0 Hz), 3.91 (s, 3H), 3.88 (s, 3H), 1.35 (t, 3H, JHH = 7.0 Hz), 1.29 (t, 3H, JHH = 7.0 Hz) ppm. 13C{1H} NMR (75 MHz, CDCl3): δ = 179.0, 166.6, 162.4, 161.5, 161.1, 160.1, 157.1, 133.5, 133.2, 132.1, 130.1, 122.9, 114.1, 113.9, 113.6, 55.5, 55.4, 43.6, 43.1, 12.5, 12.4 ppm. IR (KBr): ν = 2969 (w), 2930 (w), 2837 (w), 1683 (m), 1660 (s), 1604 (s), 1533 (s), 1451 (w), 1423 (m), 1382 (s), 1335 (s), 1281 (s), 1256 (s), 1236 (s), 1212 (s), 1172 (s), 1139 (m), 1107 (s), 1078 (m), 1025 (m), 887 (m), 832 (m), 785 (m), 661 (w), 554 (w), 491 (w) cm−1. HRMS (ESI): m/z 451.168272 [M + H+] (calcd. for C25H27O4N2S: 451.168605).
3.3. 5-(3,3-Bis(4-(dimethylamino)phenyl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (3b)
Purple solid. Yield: 0.154 g (65%). 1H NMR (300 MHz, CDCl3): δ = 8.48 (d, 1H, JHH = 13.2 Hz), 8.23 (d, 1H, JHH = 13.2 Hz), 7.50 (d, 2H, JHH = 9.0 Hz), 7.25 (d, 2H, JHH = 9.0 Hz), 6.76 (d, 2H, JHH = 9.0 Hz), 6.67 (d, 2H, JHH = 9.0 Hz), 4.62 (q, 2H, JHH = 6.9 Hz), 4.53 (q, 2H, JHH = 6.9 Hz), 3.10 (s, 6H), 3.09 (s, 6H), 1.36 (t, 3H, JHH = 6.9 Hz), 1.30 (t, 3H, JHH = 6.9 Hz) ppm. 13C{1H} NMR (75 MHz, CDCl3): δ = 178.8, 171.0, 161.8, 160.6, 158.1, 152.9, 152.4, 134.3, 133.5, 128.4, 125.7, 120.9, 111.4, 111.3, 109.4, 43.4, 42.9, 40.1, 12.6, 12.5 ppm. IR (KBr): ν = 2976 (w), 2927 (w), 1651 (s), 1592 (s), 1508 (s), 1433 (m), 1361 (s), 1283 (s), 1263 (m), 1239 (s), 1189 (s), 1142 (m), 1105 (s), 992 (w), 947 (m), 898 (m), 858 (w), 819 (m), 782 (m), 750 (w), 731 (w), 550 (w), 488 (w), 478 (w), 411 (w) cm−1. HRMS (ESI): m/z 477.231418 [M + H+] (calcd. for C27H33N4O2S: 477.231874).
3.4. 5-(3,3-Di([1,1’-biphenyl]-4-yl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (3c)
Red solid. Yield: 0.214 g (79%). 1H NMR (300 MHz, CDCl3): δ = 8.73 (d, 1H, JHH = 12.3 Hz), 8.30 (d, 1H, JHH = 12.3 Hz), 7.78–7.40 (m, 18H), 4.62 (q, 2H, JHH = 6.9 Hz), 4.54 (q, 2H, JHH = 6.9 Hz), 1.38 (t, 3H, JHH = 6.9 Hz), 1.31 (t, 3H, JHH = 6.9 Hz) ppm. 13C{1H} NMR (75 MHz, CDCl3): δ = 178.9, 165.0, 160.8, 160.0, 156.0, 143.8, 140.0, 139.5, 136.4, 131.9, 130.5, 129.0, 128.9, 128.1, 127.9, 127.3, 127.2, 127.1, 124.6, 115.3, 43.8, 43.3, 12.5, 12.4 ppm. IR (KBr): ν = 3027 (w), 2972 (w), 2930 (w), 1697 (w), 1692 (m), 1667 (s), 1598 (m), 1548 (s), 1485 (m), 1434 (m), 1386 (s), 1337 (s), 1282 (s), 1236 (m), 1212 (s), 1107 (s), 1095 (s), 1004 (m), 969 (w), 887 (w), 848 (m), 787 (w), 765 (w), 733 (w), 693 (m), 493 (w) cm−1. Elemental analysis calcd. (%) for C35H30N2O2S: C 77.46, H 5.57, N 5.16; found: C 77.29, H 5.59, N 5.25. HRMS (ESI): m/z 543.210255 [M + H+] (calcd. for C35H31N2O2S: 543.210076).
4. Conclusions
Based on the ability of InCl3 to promote the tandem Meyer–Schuster/Knoevenagel condensation of terminal propargylic alcohols with 1,3-dicarbonyl compounds in water under MW irradiation [18], a new synthesis of the previously reported butadiene dyes 3a,b has been developed. In addition, applaying the same MW-assisted InCl3-catalyzed protocol, the related butadiene 5-(3,3-bi([1,1’-biphenyl]-4-yl)allylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 3c could also be synthesized for the first time, and fully characterized, starting from 1,3-diethyl-2-thiobarbituric acid and 1,1-di([1,1’-biphenyl]-4-yl)prop-2-yn-1-ol.
Supplementary Materials
The following supporting information can be downloaded online, Figures S1–S5: 1H, 13C{1H}, DEPT-1,3,5, IR and HRMS spectra obtained for compound 3a; Figures S6–S10: 1H, 13C{1H}, DEPT-1,3,5, IR and HRMS spectra obtained for compound 3b; Figures S11–S15: 1H, 13C{1H}, DEPT-1,3,5, IR and HRMS spectra obtained for compound 3c; Figures S16 and S17: UV-Vis spectrum of compound 3c recorded in heptane and DMSO solution.
Author Contributions
Conceptualization, V.C.; synthesis and characterization of compounds 3a–c, J.F. Both authors contributed to the discussion of the experimental results as well as the writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO project CTQ2016-75896-P).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials file.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds 3a–c are not available from the authors.
References
- Meier, H. Conjugated oligomers with terminal donor-acceptor substitution. Angew. Chem. Int. Ed. 2005, 44, 2482–2506. [Google Scholar]
- Bureš, F. Fundamental aspects of property tuning in Push–Pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar]
- Beverina, L.; Pagani, G.A. π-Conjugated zwitterions as paradigm of donor-acceptor building blocks in organic based materials. Acc. Chem. Res. 2014, 47, 319–329. [Google Scholar]
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on Push–Pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar]
- Razvi, M.A.N.; Bakry, A.H.; Afzal, S.M.; Khan, S.A.; Asiri, A.M. Synthesis, characterization and determination of third-order optical nonlinearity by cw z-scan technique of novel thiobarbituric acid derivative dyes. Mater. Lett. 2015, 144, 131–134. [Google Scholar]
- Ibrahim, M.M.; El-Shafai, N.M.; El-Mehasseb, I.M.; Abdou, S.N.; El-Sheshtawy, H.S. Tuning optical properties of triphenylamine-pyrrole by alkyl-substituted thiobarbituric acid for dye-sensitized solar cell. Int. J. Energy Res. 2021, 45, 14804–14812. [Google Scholar]
- Omar, A.Z.; Mahmoud, M.N.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M.A. A combined experimental and DFT investigation of mono azo thiobarbituric acid based chalcone disperse dyes. Dye. Pigment. 2021, 185, 108887. [Google Scholar]
- Kawaura, M.; Aizawa, T.; Takahashi, S.; Miyasaka, H.; Sotome, H.; Yagai, S. Fluorescent supramolecular polymers of barbiturate dyes with thiophene-cored twisted π-systems. Chem. Sci. 2022, 13, 1281–1287. [Google Scholar]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Duval, S.; Nechab, M.; Gigmes, D.; Dumur, F. Synthesis, optical and electrochemical properties of a series of Push–Pull dyes based on the 4,4-bis(4-methoxy phenyl)butadienyl donor. Dye. Pigment. 2021, 194, 109552. [Google Scholar]
- Pigot, C.; Péralta, S.; Bui, T.-T.; Nechab, M.; Dumur, F. Push–Pull dyes based on Michler´s aldehyde: Design and characterization of the optical and electrochemical properties. Dye. Pigment. 2022, 202, 110278. [Google Scholar]
- Engel, D.A.; Dudley, G.B. The Meyer–Schuster rearrangement for the synthesis of α,β-unsaturated carbonyl compounds. Org. Bimol. Chem. 2009, 7, 4149–4158. [Google Scholar]
- Cadierno, V.; Crochet, P.; García-Garrido, S.E.; Gimeno, J. Metal-catalyzed transformations of propargylic alcohols into α,β-unsaturated carbonyl compounds: From the Meyer-Schuster and Rupe rearrangements to redox isomerizations. Dalton Trans. 2010, 39, 4015–4031. [Google Scholar]
- Justaud, F.; Hachem, A.; Grée, R. Recent developments in the Meyer-Schuster rearrangement. Eur. J. Org. Chem. 2021, 2021, 514–542. [Google Scholar]
- Cadierno, V.; Francos, J.; Gimeno, J. Microwave-assisted InCl3-catalyzed Meyer–Schuster rearrangement of propargylic aryl carbinols in aqueous media: A green approach to α,β-unsaturated carbonyl compounds. Tetrahedron Lett. 2009, 50, 4773–4776. [Google Scholar]
- Borge, J.; Cadierno, V.; Díez, J.; García-Garrido, S.E.; Gimeno, J. Novel Push–Pull butadienes derived from 1,1-diaryl-2-propyn-1-ols and 1,1,1,5,5,5-hexafluoro-2,4-pentanedione: Synthesis, absorption spectra and solvatochromic behaviour. Dye. Pigment. 2010, 87, 209–217. [Google Scholar]
- Francos, J.; García-Garrido, S.E.; Borge, J.; Suárez, F.J.; Cadierno, V. Butadiene dyes based on 3-(dicyanomethylidene)indan-1-one and 1,3-bis(dicyanomethylidene)indane: Synthesis, characterization and solvatochromic behaviour. RSC Adv. 2016, 6, 6858–6867. [Google Scholar]
- Francos, J.; Borge, J.; Díez, J.; García-Garrido, S.E.; Cadierno, V. Easy entry to donor/acceptor butadiene dyes through MW-assisted InCl3-catalyzed coupling of propargylic alcohols with indan-1,3-dione in water. Catal. Commun. 2015, 63, 10–14. [Google Scholar]
- Gabbutt, C.D.; Heron, B.M.; Instone, A.C.; Thomas, D.A.; Partington, S.M.; Hursthouse, M.B.; Gelbrich, T. Observations on the synthesis of photochromic naphthopyrans. Eur. J. Org. Chem. 2003, 2003, 1220–1230. [Google Scholar]
- Bilton, C.; Howard, J.A.K.; Madhavi, N.N.L.; Nangia, A.; Desiraju, G.R.; Allen, F.A.; Wilson, C.C. Crystal engineering in the gem-alkynol family; synthon repetitivity and topological similarity in diphenylethynylmethanols: Structures that lack O-H⋯O hydrogen bonds. Acta Cryst. 2000, B56, 1071–1079. [Google Scholar]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).