3-(2-Diisopropylaminoethyl)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione
Abstract
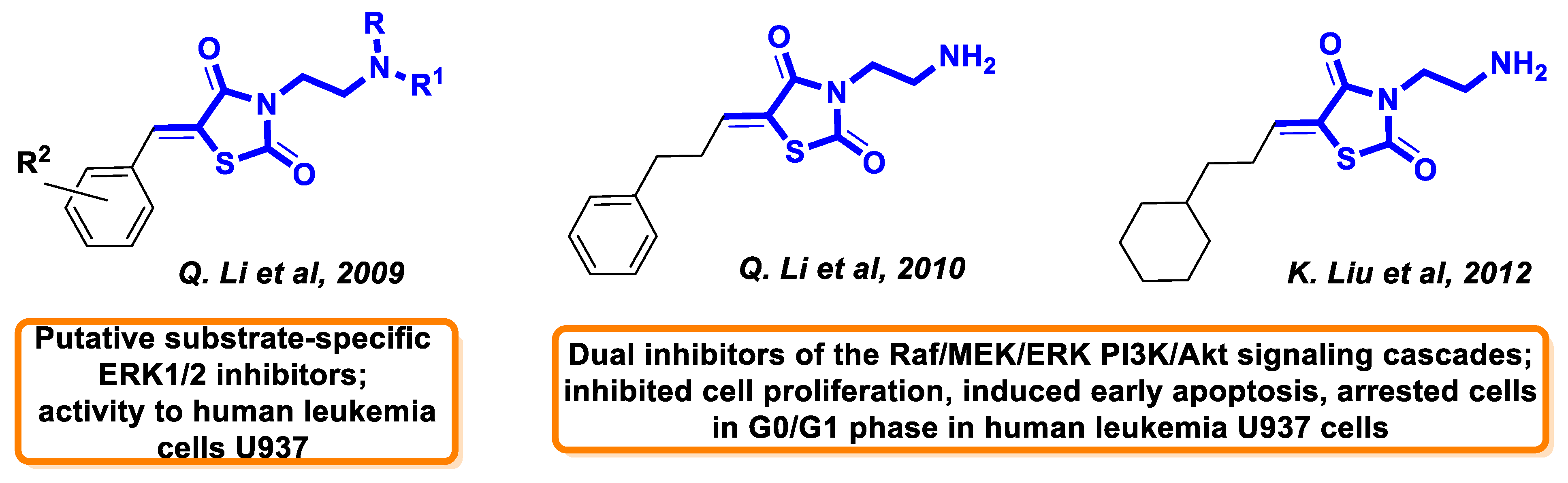
:1. Introduction
2. Results and Discussion
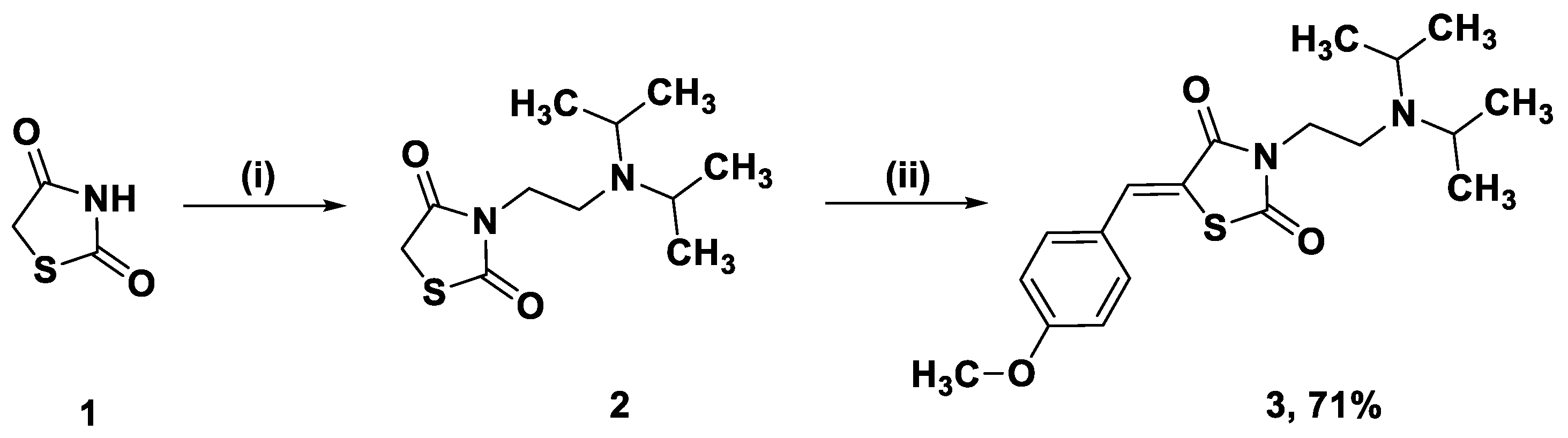
2.1. Synthesis of the Title Compound 3
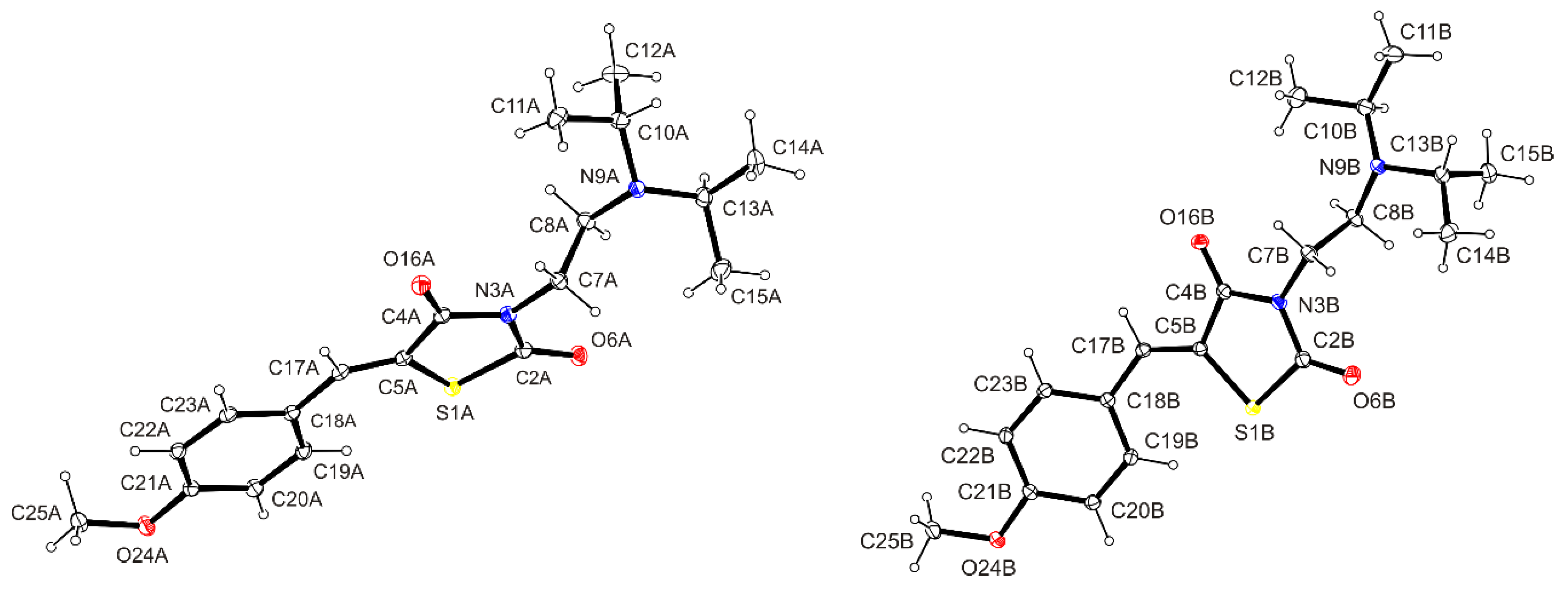
2.2. X-ray Crystallographic Study of the Title Compound 3
3. Materials and Methods
3.1. 3-(2-Diisopropylaminoethyl)thiazolidine-2,4-dione (2)
3.2. 3-(2-Diisopropylaminoethyl)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione (3)
3.3. X-ray Crystallographic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arkin, M. Protein–protein interactions and cancer: Small molecules going in for the kill. Curr. Opin. Chem. Biol. 2005, 9, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Kamkaew, A.; Burgess, K.; Kiew, L.V.; Chung, L.Y.; Lee, H.B. Small molecules for active targeting in cancer. Med. Res. Rev. 2016, 36, 494–575. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.G.; Chisholm, J.D. The next generation of immunotherapy for cancer: Small molecules could make big waves. J. Immunol. 2019, 202, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, D.E.; Velea, L.M. Intercalative binding of small molecules to nucleic acids. Curr. Org. Chem. 2000, 4, 915–929. [Google Scholar] [CrossRef]
- Holota, S.; Komykhov, S.; Sysak, S.; Gzella, A.; Cherkas, A.; Lesyk, R. Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents. Molecules 2021, 26, 1162. [Google Scholar] [CrossRef]
- Yushyn, I.; Holota, S.; Lesyk, R. 2,2-Dichloro-N-[5-[2-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]-2-oxoethyl] sulfanyl-1,3,4-thiadiazol-2-yl]acetamide. Molbank 2022, 2022, M1328. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Holota, S.; Yushyn, I.; Sabadakh, O.; Karpenko, O.; Novikov, V.; Lesyk, R. Synthesis and Biological Activity Evaluation of Polyfunctionalized Anthraquinonehydrazones. Lett. Drug Des. Discov. 2021, 18, 199–209. [Google Scholar] [CrossRef]
- Li, Q.; Al-Ayoubi, A.; Guo, T.; Zheng, H.; Sarkar, A.; Nguyen, T.; Eblen, S.T.; Grant, S.; Kellogg, G.E.; Zhang, S.H. Structure–activity relationship (SAR) studies of 3-(2-amino-ethyl)-5-(4-ethoxy-benzylidene)-thiazolidine-2,4-dione: Development of potential substrate-specific ERK1/2 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6042–6046. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zheng, H.; Liu, K.; Guo, T.L.; Liu, Y.; Eblen, S.T.; Grant, S.; Zhang, S.H. Discovery of 3-(2-aminoethyl)-5-(3-phenyl-propylidene)-thiazolidine-2,4-dione as a dual inhibitor of the Raf/MEK/ERK and the PI3K/Akt signaling pathways. Bioorg. Med. Chem. Lett. 2010, 20, 4526–4530. [Google Scholar] [CrossRef]
- Liu, K.; Rao, W.; Parikh, H.; Li, Q.; Guo, T.L.; Grant, S.; Kellogg, G.E.; Zhang, S.H. 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: Design, synthesis and biological characterization. Eur. J. Med.Chem. 2012, 47, 125–137. [Google Scholar] [CrossRef]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. Eur. J. Med. Chem. 2014, 87, 814–833. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Ottanà, R.; Curinga, C.; Vigorita, M.G.; Rakowitz, D.; Steind, T.H.; Langer, T.H. Structure–activity relationships and molecular modelling of 5-arylidene-2,4-thiazolidinediones active as aldose reductase inhibitors. Bioorg. Med. Chem. 2005, 13, 2809–2823. [Google Scholar] [CrossRef]
- Popov-Pergal, K.; Cekovic, Z.; Pergal, M. Condensation of 2,4-dioxotetrahydro-1,3-thiazole with aromatic aldehydes. J. Gen. Chem. USSR 1991, 61, 1958–1962. [Google Scholar]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Turkevych, N.M.; Vvedenskij, V.M.; Petlichnaya, L.P. Method of obtaining pseudothiohydantoin and thiazolidinedione-2,4. Ukr. Khim. Zh. 1961, 27, 680–681, reprinted in Chem. Abstr. 1962, 56, 73455. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]

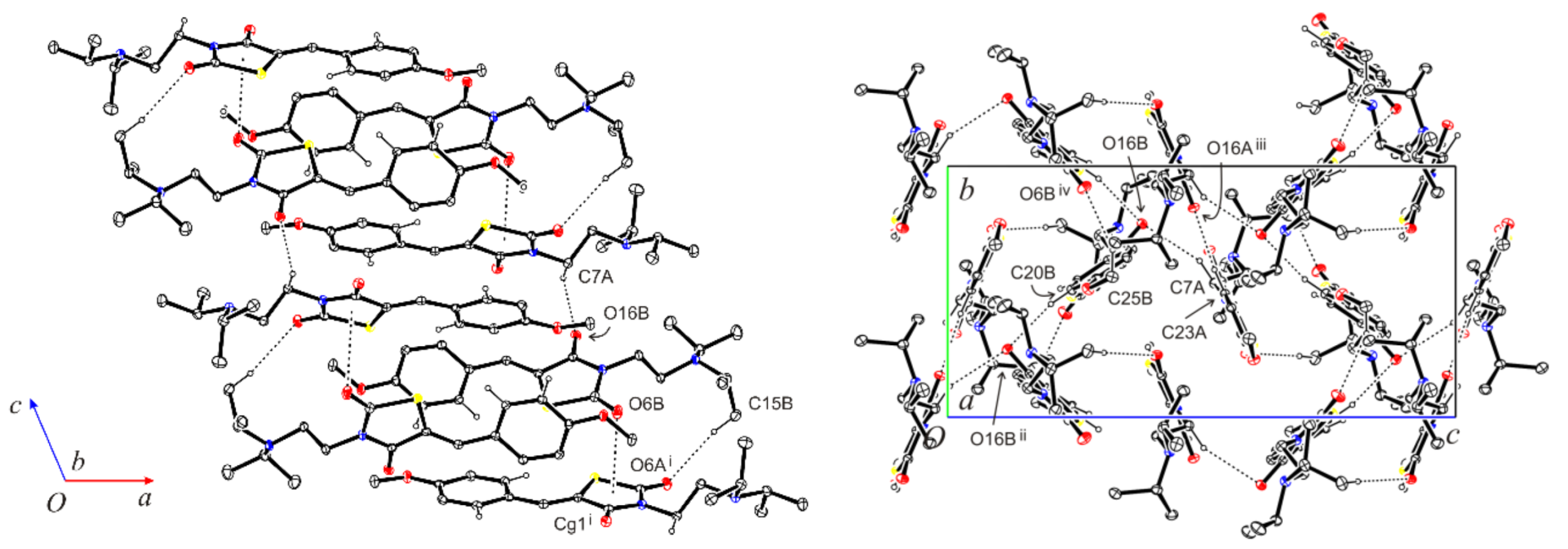

| D—H∙∙∙A | D—H (Å) | H∙∙∙A (Å) | D∙∙∙A (Å) | D—H∙∙∙A (°) |
|---|---|---|---|---|
| N7A-H7A∙∙∙O16B | 0.99 | 2.55 | 3.4291(14) | 149 |
| C15B-H15D∙∙∙O6A i | 0.98 | 2.60 | 3.5659(17) | 169 |
| C19A-H19A∙∙∙S1A | 0.95 | 2.59 | 3.2963(17) | 132 |
| C19B-H19B∙∙∙S1B | 0.95 | 2.63 | 3.3017(17) | 128 |
| C20B-H20B∙∙∙O16B ii | 0.95 | 2.50 | 3.4307(14) | 166 |
| C23A-H23A∙∙∙O16A iii | 0.95 | 2.43 | 3.3489(14) | 164 |
| C25B-H25E∙∙∙O6B iv | 0.98 | 2.56 | 3.4543(16) | 151 |
| Y—X∙∙∙Cg | Y—X (Å) | X∙∙∙Cg (Å) | Y∙∙∙Cg (Å) | Y—X∙∙∙Cg (°) |
| C2B=O6B∙∙∙Cg1 i | 1.2087(15) | 3.4383(10) | 3.7847(12) | 97.20(7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holota, S.; Yushyn, I.; Gzella, A.; Lesyk, R. 3-(2-Diisopropylaminoethyl)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione. Molbank 2022, 2022, M1394. https://doi.org/10.3390/M1394
Holota S, Yushyn I, Gzella A, Lesyk R. 3-(2-Diisopropylaminoethyl)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione. Molbank. 2022; 2022(3):M1394. https://doi.org/10.3390/M1394
Chicago/Turabian StyleHolota, Serhii, Ihor Yushyn, Andrzej Gzella, and Roman Lesyk. 2022. "3-(2-Diisopropylaminoethyl)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione" Molbank 2022, no. 3: M1394. https://doi.org/10.3390/M1394
APA StyleHolota, S., Yushyn, I., Gzella, A., & Lesyk, R. (2022). 3-(2-Diisopropylaminoethyl)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione. Molbank, 2022(3), M1394. https://doi.org/10.3390/M1394