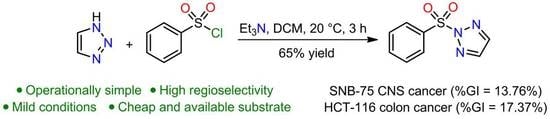
Synthesis, Spectroscopic Analysis, and In Vitro Anticancer Evaluation of 2-(Phenylsulfonyl)-2H-1,2,3-triazole
Abstract
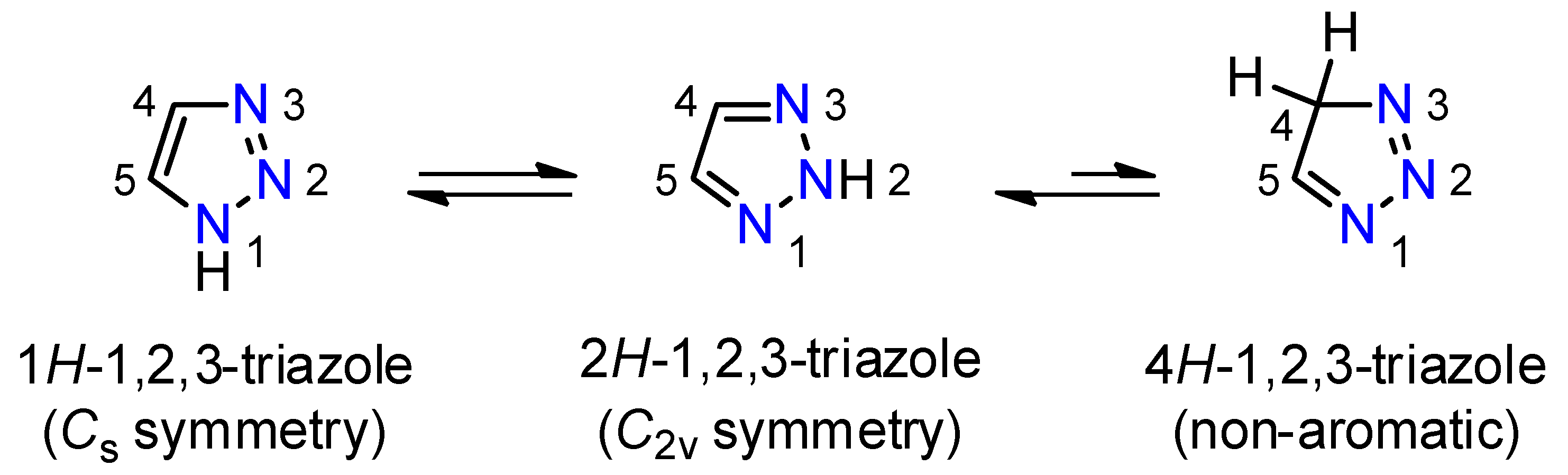
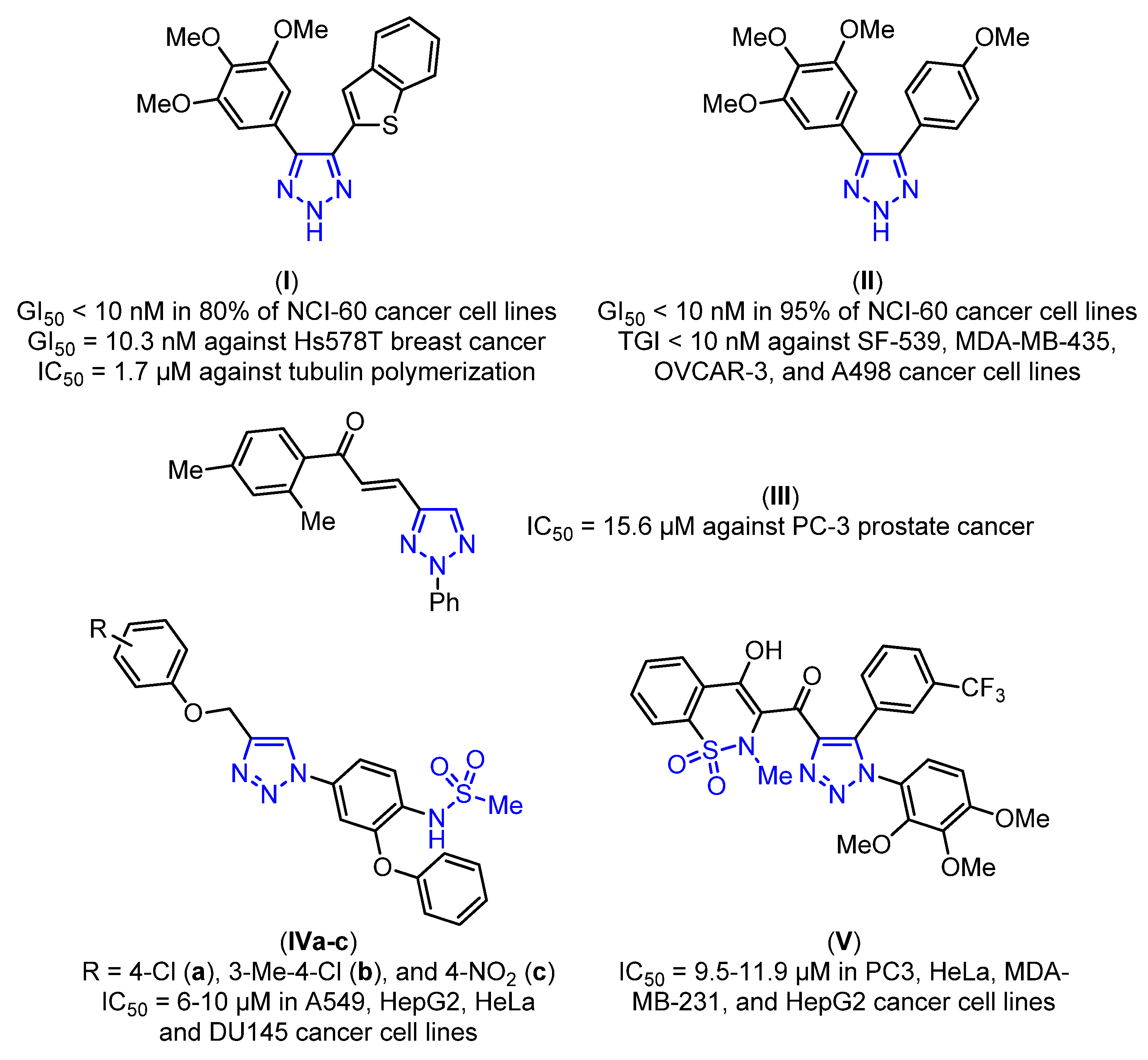
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. NMR Analysis
2.3. FTIR Analysis
2.3.1. SO2 Vibrations
2.3.2. S–N and S–C Vibrations
2.3.3. C=C Vibrations
2.3.4. N–N, C–N and C=N Vibrations
2.3.5. C–H Vibrations
2.4. UV–Vis Analysis
2.5. In Vitro Anticancer Evaluation
3. Materials and Methods
3.1. General Information
3.2. 2-(Phenylsulfonyl)-2H-1,2,3-triazole 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Nasri, S.; Bayat, M.; Kochia, K. Strategies for synthesis of 1,2,4-triazole-containing scaffolds using 3-amino-1,2,4-triazole. Mol. Divers. 2022, 26, 717–739. [Google Scholar] [CrossRef]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Hadda, T.B.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and their derivatives: Chemistry, synthesis, and therapeutic applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Lunazzi, L.; Parisi, F.; Macciantelli, D. Conformational studies by dynamic nuclear magnetic resonance spectroscopy. Part 27. Kinetics and mechanism of annular tautomerism in isomeric triazoles. J. Chem. Soc. Perkin Trans. 1984, 2, 1025–1028. [Google Scholar] [CrossRef]
- Begtrup, M.; Nielsen, C.J.; Nygaard, L.; Samdal, S.; Sjøgren, C.E.; Sørensen, G.O. The molecular structure and tautomer equilibrium of gaseous 1,2,3-triazole studied by microwave spectroscopy, electron diffraction, and ab initio calculations. Acta Chem. Scand. B Org. Chem. Biochem. 1988, 42, 500–514. [Google Scholar] [CrossRef] [Green Version]
- Catalán, J.; Sánchez-Cabezudo, M.; De Paz, J.L.G.; Elguero, J.; Taft, R.W.; Anvia, F. The tautomerism of 1,2,3-triazole, 3(5)-methylpyrazole and their cations. J. Comput. Chem. 1989, 10, 426–433. [Google Scholar] [CrossRef]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide bond bioisosteres: Strategies, synthesis, and successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H-1,2,3-triazoles. Eur. J. Med. Chem. 2021, 212, 113069. [Google Scholar] [CrossRef]
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-containing compounds as anti–lung cancer agents: Current developments, mechanisms of action, and structure–activity relationship. Front. Pharmacol. 2021, 12, 661173. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Penthala, N.R.; Madhukuri, L.; Thakkar, S.; Madadi, N.R.; Lamture, G.; Eoff, R.L.; Crooks, P.A. Synthesis and anti-cancer screening of novel heterocyclic-(2H)-1,2,3-triazoles as potential anti-cancer agents. Med. Chem. Commun. 2015, 6, 1535–1543. [Google Scholar] [CrossRef] [Green Version]
- Madadi, N.R.; Penthala, N.R.; Howk, K.; Ketkar, A.; Eoff, R.L.; Borrelli, M.J.; Crooks, P.A. Synthesis and biological evaluation of novel 4,5-disubstituted 2H-1,2,3-triazoles as cis-constrained analogues of combretastatin A-4. Eur. J. Med. Chem. 2015, 103, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, S.; Pessôa, J.C.; Pinheiro, E.M.C.; Muri, E.M.F.; Filho, E.V.; Loureiro, L.B.; Freitas, M.C.R.; Silva Junior, C.M.D.; Fiorot, R.G.; Carneiro, J.W.M.; et al. 2H-1,2,3-Triazole-chalcones as novel cytotoxic agents against prostate cancer. Bioorg. Med. Chem. Lett. 2020, 30, 127454. [Google Scholar] [CrossRef]
- Mareddy, J.; Suresh, N.; Kumar, C.G.; Kapavarapu, R.; Jayasree, A.; Pal, S. 1,2,3-Triazole-nimesulide hybrid: Their design, synthesis and evaluation as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 518–523. [Google Scholar] [CrossRef]
- Swaroop, D.K.; Kumar, N.R.; Ratnakarreddy, K.; Raja, G.; Srigiridhar, K.; Poornachandra, Y.; Kumar, C.G.; Babu, N.J.; Kumar, G.S.; Narsaiah, B. Novel 1,2,3-triazole-functionalized 1,2-benzothiazine 1,1-dioxide derivatives: Regioselective synthesis, biological evaluation and docking studies. ChemistrySelect 2018, 3, 2398–2403. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Bahrami, K.; Hakimpoor, F. High yielding protocol for direct conversion of thiols to sulfonyl chlorides and sulfonamides. J. Sulfur Chem. 2019, 40, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, K.; Khodaei, M.M.; Soheilizad, M. Direct conversion of thiols to sulfonyl chlorides and sulfonamides. J. Org. Chem. 2009, 74, 9287–9291. [Google Scholar] [CrossRef]
- Chen, Y.; Murray, P.R.D.; Davies, A.T.; Willis, M.C. Direct copper-catalyzed three-component synthesis of sulfonamides. J. Am. Chem. Soc. 2018, 140, 8781–8787. [Google Scholar] [CrossRef]
- Laudadio, G.; Barmpoutsis, E.; Schotten, C.; Struik, L.; Govaerts, S.; Browne, D.L.; Noël, T. Sulfonamide synthesis through electrochemical oxidative coupling of amines and thiols. J. Am. Chem. Soc. 2019, 141, 5664–5668. [Google Scholar] [CrossRef] [Green Version]
- Elejalde, N.R.; Macías, M.; Castillo, J.-C.; Sortino, M.; Svetaz, L.; Zacchino, S.; Portilla, J. Synthesis and in vitro antifungal evaluation of novel N-substituted 4-aryl-2-methylimidazoles. ChemistrySelect. 2018, 3, 5220–5227. [Google Scholar] [CrossRef]
- Moreno-Fuquen, R.; Arango-Daraviña, K.; Becerra, D.; Castillo, J.C.; Kennedy, A.R.; Macías, M.A. Catalyst- and solvent-free synthesis of 2-fluoro-N-(3-methyl sulfanyl-1H-1,2,4-triazol-5-yl)benzamide through a microwave-assisted Fries rearrangement: X-ray structural and theoretical studies. Acta Cryst. 2019, C75, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.-C.; Bravo, N.-F.; Tamayo, L.-V.; Mestizo, P.-D.; Hurtado, J.; Macías, M.; Portilla, J. Water-compatible synthesis of 1,2,3-triazoles under ultrasonic conditions by a Cu(I) complex-mediated click reaction. ACS Omega 2020, 5, 30148–30159. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Lan, X.; Yang, J.Z.; Denisko, O.V. Properties and synthetic utility of N-substituted benzotriazoles. Chem. Rev. 1998, 98, 409–548. [Google Scholar] [CrossRef]
- Yamauchi, M.; Miura, T.; Murakami, M. Preparation of 2-sulfonyl-1,2,3-triazoles by base-promoted 1,2-rearrangement of a sulfonyl group. Heterocycles 2010, 80, 177–181. [Google Scholar] [CrossRef]
- Keith, J.M. One-step conversion of azine N-oxides to α-1,2,4-triazolo-,1,2,3-triazolo, imidazolo-, and pyrazoloheteroarenes. J. Org. Chem. 2010, 75, 2722–2725. [Google Scholar] [CrossRef]
- Jie, K.; Wang, Y.; Huang, L.; Guo, S.; Cai, H. Convenient sulfonylation of imidazoles and triazoles using NFSI. J. Sulphur Chem. 2018, 39, 465–471. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Sambathkumar, K. Molecular structure and vibrational spectra of 2,4,6-trimethylbenzenesulphonyl chloride (FTIR & Raman) by quantum chemical calculations. Indian J. Pure Appl. Phys. 2020, 58, 589–598. [Google Scholar]
- Gökce, H.; Şen, F.; Sert, Y.; Abdel-Wahab, B.F.; Kariuki, B.M.; El-Hiti, G.A. Quantum computational investigation of (E)-1-(4-methoxyphenyl)-5-methyl-N′-(3-phenoxybenzylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molecules 2022, 27, 2193. [Google Scholar] [CrossRef]
- Toernkvist, C.; Bergman, J.; Liedberg, B. Correlated ab initio geometries and vibrations of 1H- and 2H-1,2,3-triazole. J. Phys. Chem. 1991, 95, 3123–3128. [Google Scholar] [CrossRef]
- Kudchadker, S.A.; Rao, C.N.R. Infrared spectra & normal vibrations of isomeric triazoles. Indian J. Chem. 1973, 11, 140–142. [Google Scholar]
- Billes, F.; Endrédi, H.; Keresztury, G. Vibrational spectroscopy of triazoles and tetrazoles. J. Mol. Struct. Theochem. 2000, 530, 183–200. [Google Scholar] [CrossRef]
- King, G.A.; Oliver, T.A.A.; Nix, M.G.D.; Ashfold, M.N.R. Exploring the mechanisms of H atom loss in simple azoles: Ultraviolet photolysis of pyrazole and triazole. J. Chem. Phys. 2010, 132, 064305. [Google Scholar] [CrossRef]
- Samir, B.; Kalalian, C.; Roth, E.; Salghi, R.; Chakir, A. Gas-phase UV absorption spectra and OH-oxidation kinetics of 1H-1,2,3-triazole and pyrazole. RSC Adv. 2019, 9, 27361–27368. [Google Scholar] [CrossRef] [Green Version]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.; Vélez, I.D.; Upegui, Y.; Nogueras, M.; Cobo, J. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl)amino]chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Jiménez, E.; Portilla, J.; Insuasty, B.; Quiroga, J.; Moreno-Fuquen, R.; Kennedy, A.R.; Abonia, R. Application of a catalyst-free Domino Mannich/Friedel-Crafts alkylation reaction for the synthesis of novel tetrahydroquinolines of potential antitumor activity. Tetrahedron 2018, 74, 932–947. [Google Scholar] [CrossRef]
- Serrano-Sterling, C.; Becerra, D.; Portilla, J.; Rojas, H.; Macías, M.; Castillo, J.-C. Synthesis, biological evaluation and X-ray crystallographic analysis of novel (E)-2-cyano-3-(het)arylacrylamides as potential anticancer agents. J. Mol. Struct. 2021, 1244, 130944. [Google Scholar] [CrossRef]
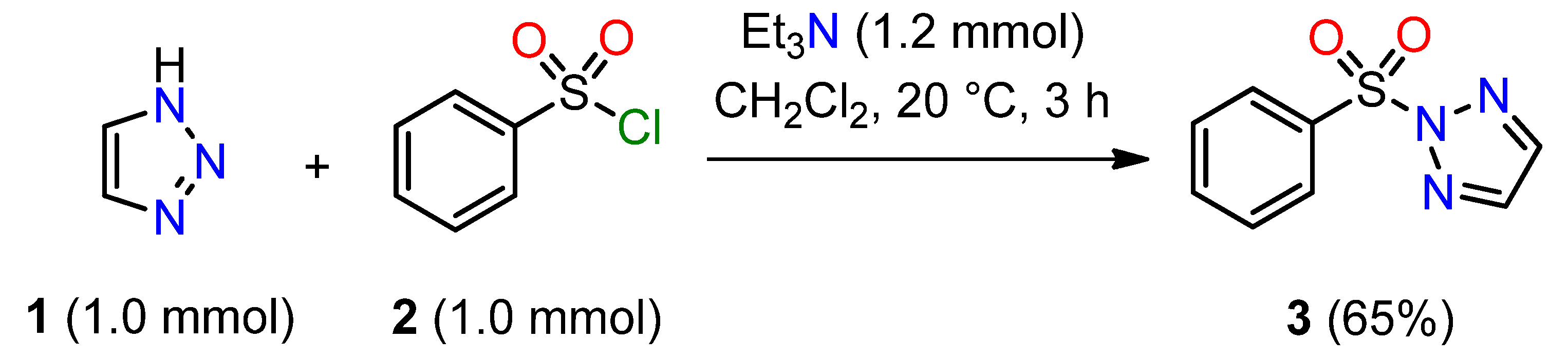
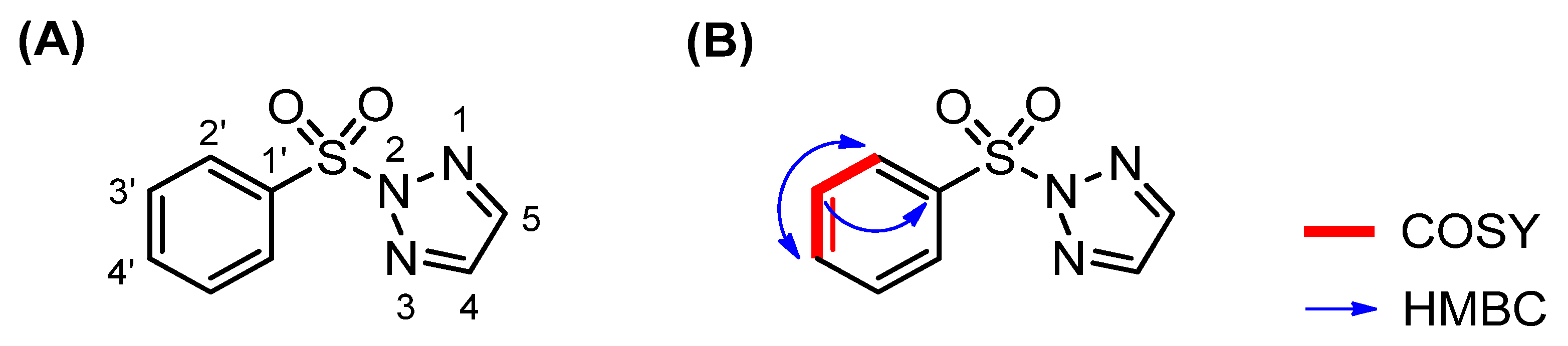
| Number | δH (mult, J in Hz) | δC (ppm) | COSY (1H-1H) | NOESY (1H-1H) | HMBC (1H-13C) |
|---|---|---|---|---|---|
| 4 and 5 | 7.85 (s) | 138.5 | — | — | — |
| 1′ | — | 136.1 | — | — | H-3′ (3J) |
| 2′ | 8.10 (dd, J = 7.8, 1.2) | 128.8 | H-3′ (3J) | H-3′ | H-4′ (3J) |
| 3′ | 7.56 (dd, J = 7.8, 7.8) | 129.7 | H-2′ (3J) H-4′ (3J) | H-2′ H-4′ | — |
| 4′ | 7.68 (tt, J = 7.8, 1.2) | 135.4 | H-3′ (3J) | H-3′ | H-2′ (3J) |
| Mean Growth | Most Sensitive Cell Line | Growth Inhibition Percentage (GI%) a |
|---|---|---|
| 100.60 | UO-31 (Renal Cancer) | 10.83 |
| SNB-75 (CNS Cancer) | 13.76 | |
| HCT-116 (Colon Cancer) | 17.37 | |
| BT-549 (Breast Cancer) | 17.64 | |
| RXF 393 (Renal Cancer) | −12.66 b | |
| OVCAR-3 (Ovarian Cancer) | −15.85 b | |
| HOP-92 (Non-Small Cell Lung Cancer) | −27.59 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas-Torres, A.; Portilla, J.; Rojas, H.; Becerra, D.; Castillo, J.-C. Synthesis, Spectroscopic Analysis, and In Vitro Anticancer Evaluation of 2-(Phenylsulfonyl)-2H-1,2,3-triazole. Molbank 2022, 2022, M1387. https://doi.org/10.3390/M1387
Salinas-Torres A, Portilla J, Rojas H, Becerra D, Castillo J-C. Synthesis, Spectroscopic Analysis, and In Vitro Anticancer Evaluation of 2-(Phenylsulfonyl)-2H-1,2,3-triazole. Molbank. 2022; 2022(2):M1387. https://doi.org/10.3390/M1387
Chicago/Turabian StyleSalinas-Torres, Angélica, Jaime Portilla, Hugo Rojas, Diana Becerra, and Juan-Carlos Castillo. 2022. "Synthesis, Spectroscopic Analysis, and In Vitro Anticancer Evaluation of 2-(Phenylsulfonyl)-2H-1,2,3-triazole" Molbank 2022, no. 2: M1387. https://doi.org/10.3390/M1387
APA StyleSalinas-Torres, A., Portilla, J., Rojas, H., Becerra, D., & Castillo, J.-C. (2022). Synthesis, Spectroscopic Analysis, and In Vitro Anticancer Evaluation of 2-(Phenylsulfonyl)-2H-1,2,3-triazole. Molbank, 2022(2), M1387. https://doi.org/10.3390/M1387