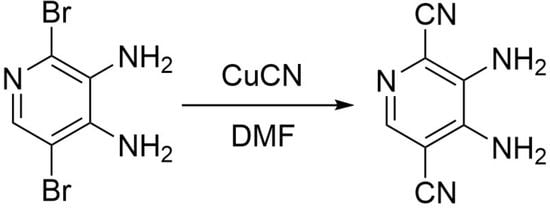
3,4-Diaminopyridine-2,5-dicarbonitrile
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
- Synthesis of 3,4-diaminopyridine-2,5-dicarbonitrile 2 (Supplementary Materials).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rakitin, O.A. Recent Developments in the Synthesis of 1,2,5-Thiadiazoles and 2,1,3-Benzothiadiazoles. Synthesis 2019, 51, 4338–4347. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Rakitin, O.A. Recent Developments in the Synthesis and Applications of 1,2,5-Thia- and Selenadiazoles. A Review. Org. Prep. Proc. Int. 2014, 46, 475–544. [Google Scholar] [CrossRef]
- Sato, N. Pyrazines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; pp. 273–331. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Rashed, N. Bicyclic 6-6 Systems: Three Heteroatoms 1:2. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; pp. 759–845. [Google Scholar] [CrossRef]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, Art.481. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Mikhailov, M.S.; Gudim, N.S.; Knyazeva, E.A.; Tanaka, E.; Zhang, L.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole—A new donor building-block in the design of sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A 2020, 391, 112333. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle light-style OLEDs with benzochalcogenadiazoles cores. Dyes Pigm. 2021, 185, 108917. [Google Scholar] [CrossRef]
- Leventis, A.; Chmovzh, T.N.; Knyazeva, E.A.; Han, Y.; Heeney, M.J.; Rakitin, O.A.; Bronstein, H. A novel low-bandgap pyridazine thiadiazole-based conjugated polymer with deep molecular orbital levels. Polym. Chem. 2020, 11, 581–585. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Zhang, Y.; Chen, K.-S.; Zeigler, D.F.; Chen, F.-C.; Lin, B.; Jen, A.K.-Y. High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo[3,4-c]pyridine units for thin film transistor and photovoltaic applications. J. Mater. Chem. 2011, 21, 13247–13255. [Google Scholar] [CrossRef]
- Hou, Z.; Suzuki, Y.; Oishi, S.; Fujii, N.; Ohno, H. Efficient synthesis of aminomethylated azaindoles and corresponding pyrrole-fused derivatives by copper-catalyzed domino multicomponent coupling and cyclization. Tetrahedron 2012, 68, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Lindley, J. Tetrahedron report number 163. Tetrahedron 1984, 40, 1433–1456. [Google Scholar] [CrossRef]
- Yang, C.; Williams, J.M. Palladium-Catalyzed Cyanation of Aryl Bromides Promoted by Low-Level Organotin Compounds. Org. Lett. 2004, 6, 2837–2840. [Google Scholar] [CrossRef] [PubMed]
- Schareina, T.; Zapf, A.; Mägerlein, W.; Müller, N.; Beller, M. A State-of-the-Art Cyanation of Aryl Bromides: A Novel and Versatile Copper Catalyst System Inspired by Nature. Chem. Eur. J. 2007, 13, 6249–6254. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Brun, R.; Easterbrook, J.D.; Tanious, F.A.; Wilson, W.D.; Boykin, D.W. Synthesis and Antiprotozoal Activity of Aza-Analogues of Furamidine. J. Med. Chem. 2003, 46, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, E.; Agama, K.; Pommier, Y.; Cushman, M. Azaindenoisoquinolines as Topoisomerase I Inhibitors and Potential Anticancer Agents: A Systematic Study of Structure–Activity Relationships. J. Med. Chem. 2012, 55, 1682–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, W.B.; Choi, S.H.; Park, J.-Y.; Choi, S.H.; Finn, J.; Yoon, S.-H. Discovery of torezolid as a novel 5-hydroxymethyl-oxazolidinone antibacterial agent. Eur. J. Med. Chem. 2011, 46, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
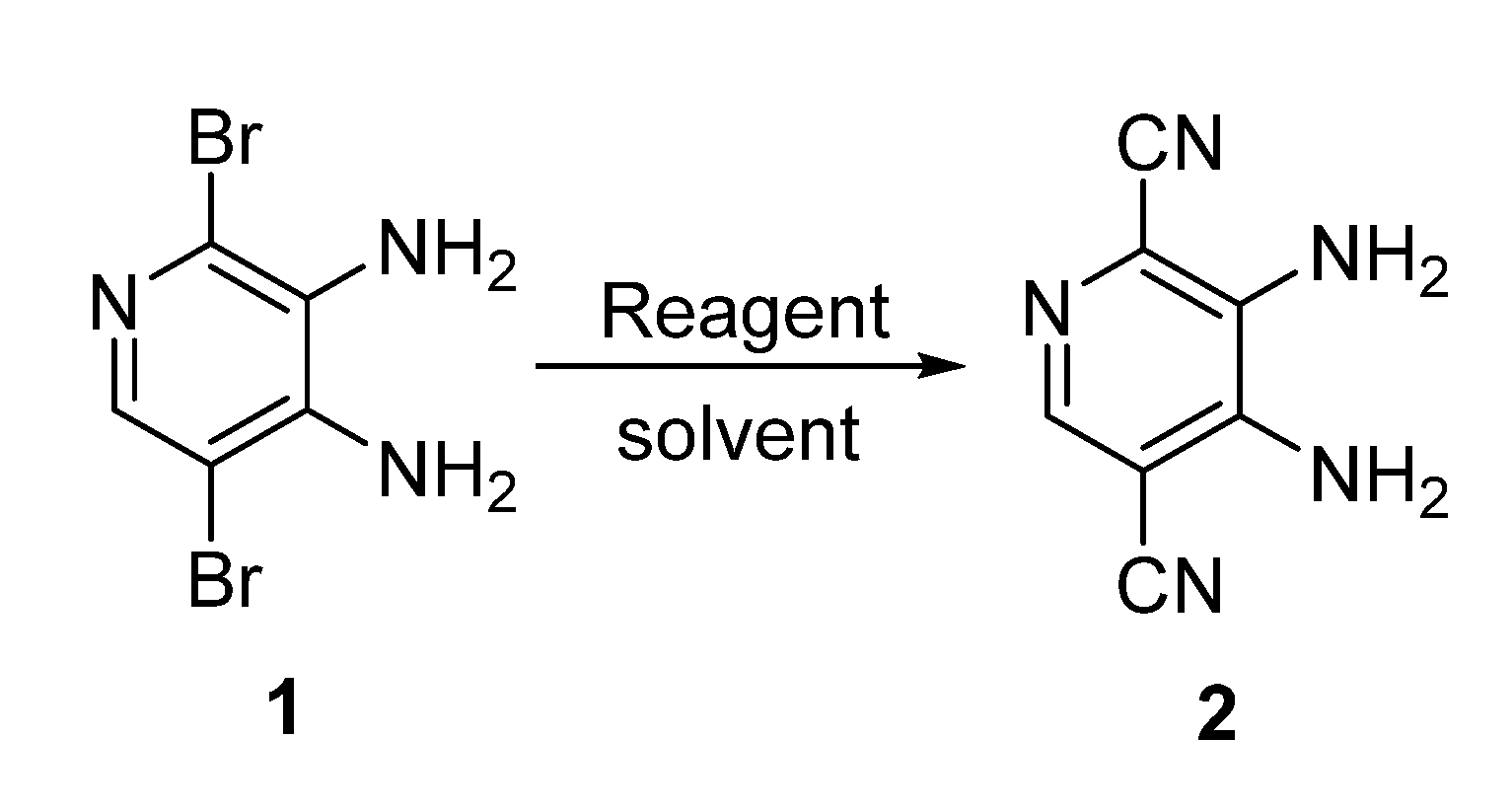
| Entry | Solvent | Reagent | Temperature, °C | Time, h | Yield of 2, % |
|---|---|---|---|---|---|
| 1 | EtOH | KCN | 78 | 10 | 0 |
| 2 | EtOH | CuCN | 78 | 10 | 0 |
| 3 | DMF | KCN | 25 | 8 | 0 |
| 4 | DMF | KCN | 60 | 8 | 0 |
| 5 | DMF | CuCN | 25 | 8 | 0 |
| 6 | DMF | CuCN | 60 | 8 | 0 |
| 7 | DMF | KCN | 100 | 8 | 0 |
| 8 | DMF | CuCN | 100 | 8 | traces |
| 9 | DMF | KCN | 120 | 8 | 0 |
| 10 | DMF | CuCN | 140 | 8 | 40 |
| 11 | DMF | CuCN | 140 | 10 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Rakitin, O.A. 3,4-Diaminopyridine-2,5-dicarbonitrile. Molbank 2022, 2022, M1386. https://doi.org/10.3390/M1386
Chmovzh TN, Rakitin OA. 3,4-Diaminopyridine-2,5-dicarbonitrile. Molbank. 2022; 2022(2):M1386. https://doi.org/10.3390/M1386
Chicago/Turabian StyleChmovzh, Timofey N., and Oleg A. Rakitin. 2022. "3,4-Diaminopyridine-2,5-dicarbonitrile" Molbank 2022, no. 2: M1386. https://doi.org/10.3390/M1386
APA StyleChmovzh, T. N., & Rakitin, O. A. (2022). 3,4-Diaminopyridine-2,5-dicarbonitrile. Molbank, 2022(2), M1386. https://doi.org/10.3390/M1386