(E)-7-(4-(Diphenylamino)styryl)benzo[c][1,2,5]thiadiazole-4-carbonitrile
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rakitin, O.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry IV; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–406. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Rakitin, O.A. Recent Developments in the Synthesis of 1,2,5-Thiadiazoles and 2,1,3-Benzothiadiazoles. Synthesis 2019, 51, 4338–4347. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Axelsson, M.; Marchiori, C.F.N.; Huang, P.; Araujo, C.M.; Tian, H. Small Organic Molecule Based on Benzothiadiazole for Electrocatalytic Hydrogen Production. J. Am. Chem. Soc. 2021, 143, 21229–21233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bauer, N.E.; Kanal, I.Y.; You, W.; Hutchison, G.R.; Meyer, T.Y. Sequence Effects in Donor–Acceptor Oligomeric Semiconductors Comprising Benzothiadiazole and Phenylenevinylene Monomers. Macromolecules 2017, 50, 151–161. [Google Scholar] [CrossRef]
- Thooft, A.M.; Cassaidy, K.; VanVeller, B. A Small Push–Pull Fluorophore for Turn-on Fluorescence. J. Org. Chem. 2017, 82, 8842–8847. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Tsai, C.-H.; Lin, F.; Huang, T.-W.; Chou, S.-H.; Wu, C.-C.; Wong, K.-T. 2,1,3-Benzothiadiazole-containing donor–acceptor–acceptor dyes for dye-sensitized solar cells. Tetrahedron 2012, 68, 7509–7516. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Huang, C.-C.; Kumar, S.; Li, S.-H.; Dong, Z.-L.; Fung, M.-K.; Jiang, Z.-Q.; Liao, L.-S. Thermally activated delayed fluorescence sensitizer for D–A–A type emitters with orange-red light emission. J. Mater. Chem. C 2018, 6, 10030–10035. [Google Scholar] [CrossRef]
- He, C.; He, Q.; Yi, Y.; Wu, G.; Bai, F.; Shuai, Z.; Li, Y. Improving the efficiency of solution processable organic photovoltaic devices by a star-shaped molecular geometry. J. Mater. Chem. 2008, 18, 4085–4090. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Rakitin, O.A. (E)-4-(2-(7-Bromo-[1,2,5]thiadiazolo[3,4-c]pyridin-4-yl)vinyl)-N,N-diphenylaniline. Molbank 2022, 2022, M1368. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, Y.; Chi, S.; Qian, X. Isomeric Boron−Fluorine Complexes with Donor−Acceptor Architecture: Strong Solid/Liquid Fluorescence and Large Stokes Shift. Org. Lett. 2008, 10, 633–636. [Google Scholar] [CrossRef] [PubMed]
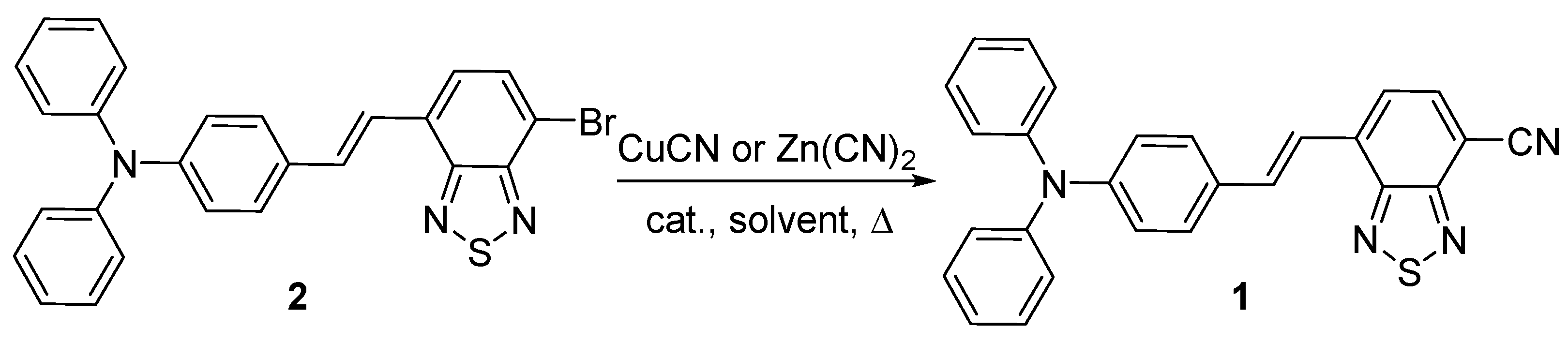
| Entry | Reagent | Catalyst. | Solvent | Temperature, °C | Time, h | Yield, of 1% |
|---|---|---|---|---|---|---|
| 1 | CuCN | - | DMF | 80 | 24 | 15 |
| 2 | CuCN | - | DMF | 120 | 28 | 40 |
| 3 | Zn(CN)2 | Pd(PPh3)4 | NMP | 80 | 16 | 20 |
| 4 | Zn(CN)2 | Pd(PPh3)4 | NMP | 120 | 24 | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Kudryashev, T.A.; Rakitin, O.A. (E)-7-(4-(Diphenylamino)styryl)benzo[c][1,2,5]thiadiazole-4-carbonitrile. Molbank 2022, 2022, M1385. https://doi.org/10.3390/M1385
Chmovzh TN, Kudryashev TA, Rakitin OA. (E)-7-(4-(Diphenylamino)styryl)benzo[c][1,2,5]thiadiazole-4-carbonitrile. Molbank. 2022; 2022(2):M1385. https://doi.org/10.3390/M1385
Chicago/Turabian StyleChmovzh, Timofey N., Timofey A. Kudryashev, and Oleg A. Rakitin. 2022. "(E)-7-(4-(Diphenylamino)styryl)benzo[c][1,2,5]thiadiazole-4-carbonitrile" Molbank 2022, no. 2: M1385. https://doi.org/10.3390/M1385
APA StyleChmovzh, T. N., Kudryashev, T. A., & Rakitin, O. A. (2022). (E)-7-(4-(Diphenylamino)styryl)benzo[c][1,2,5]thiadiazole-4-carbonitrile. Molbank, 2022(2), M1385. https://doi.org/10.3390/M1385








