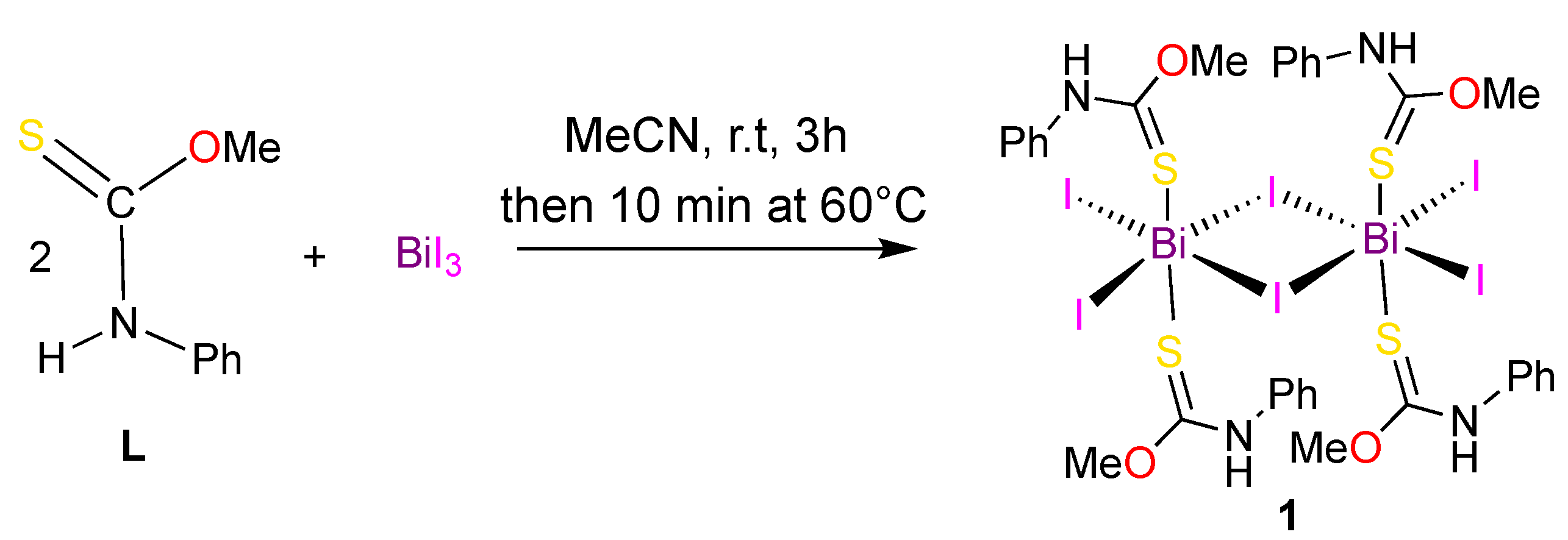
Bis(μ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth
Abstract
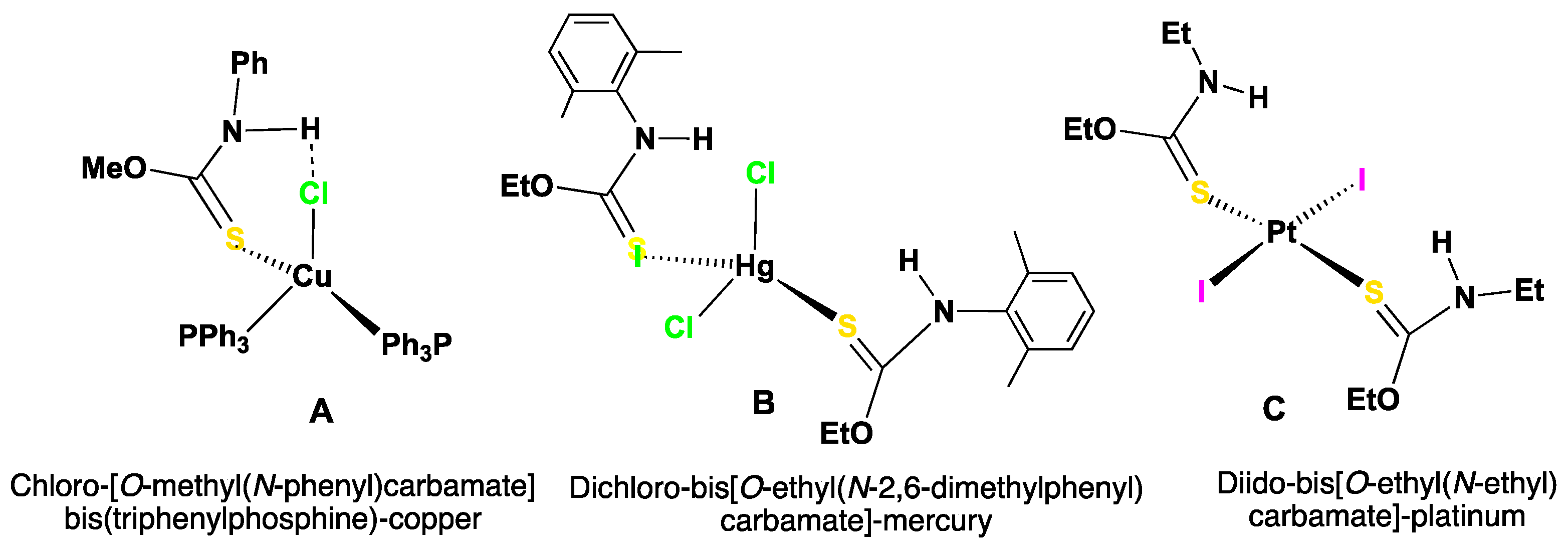
1. Introduction
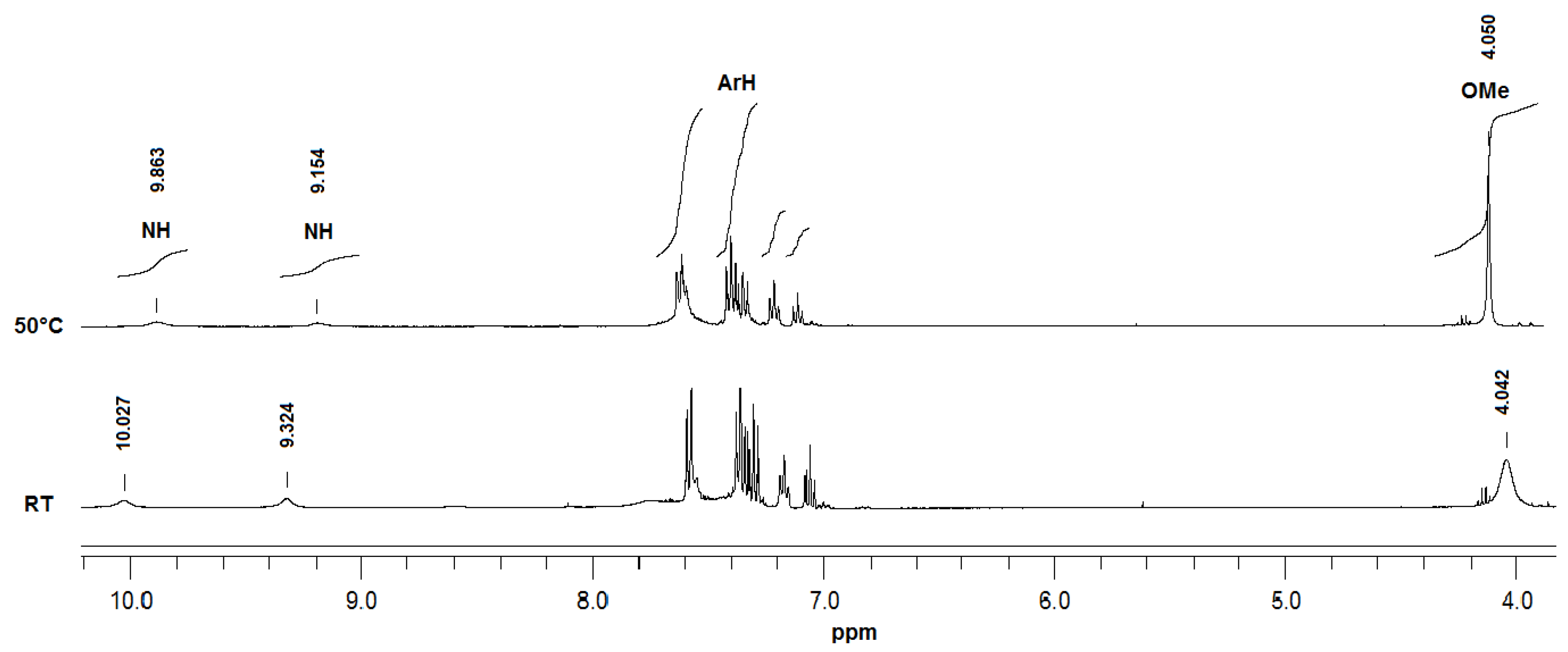
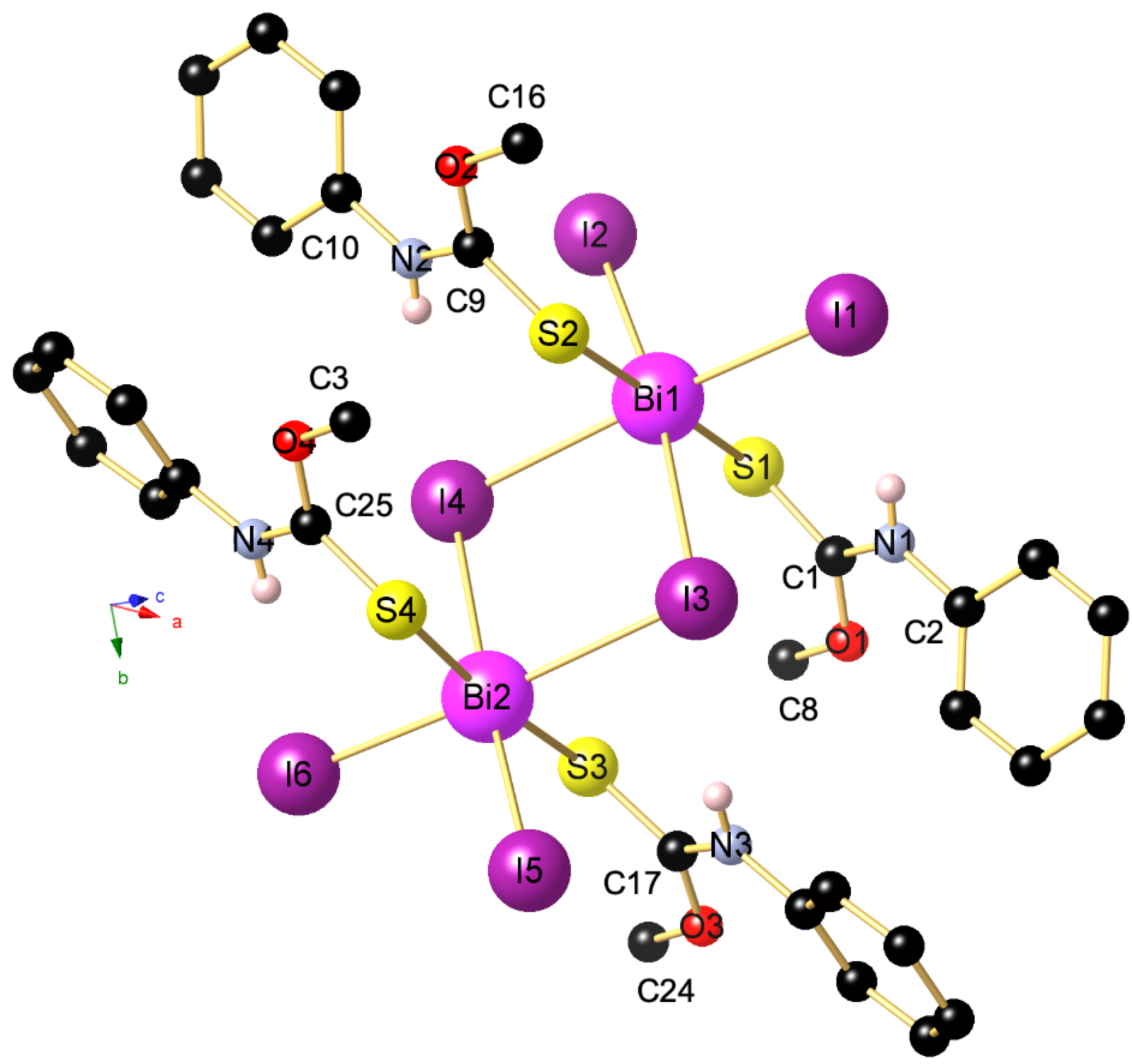
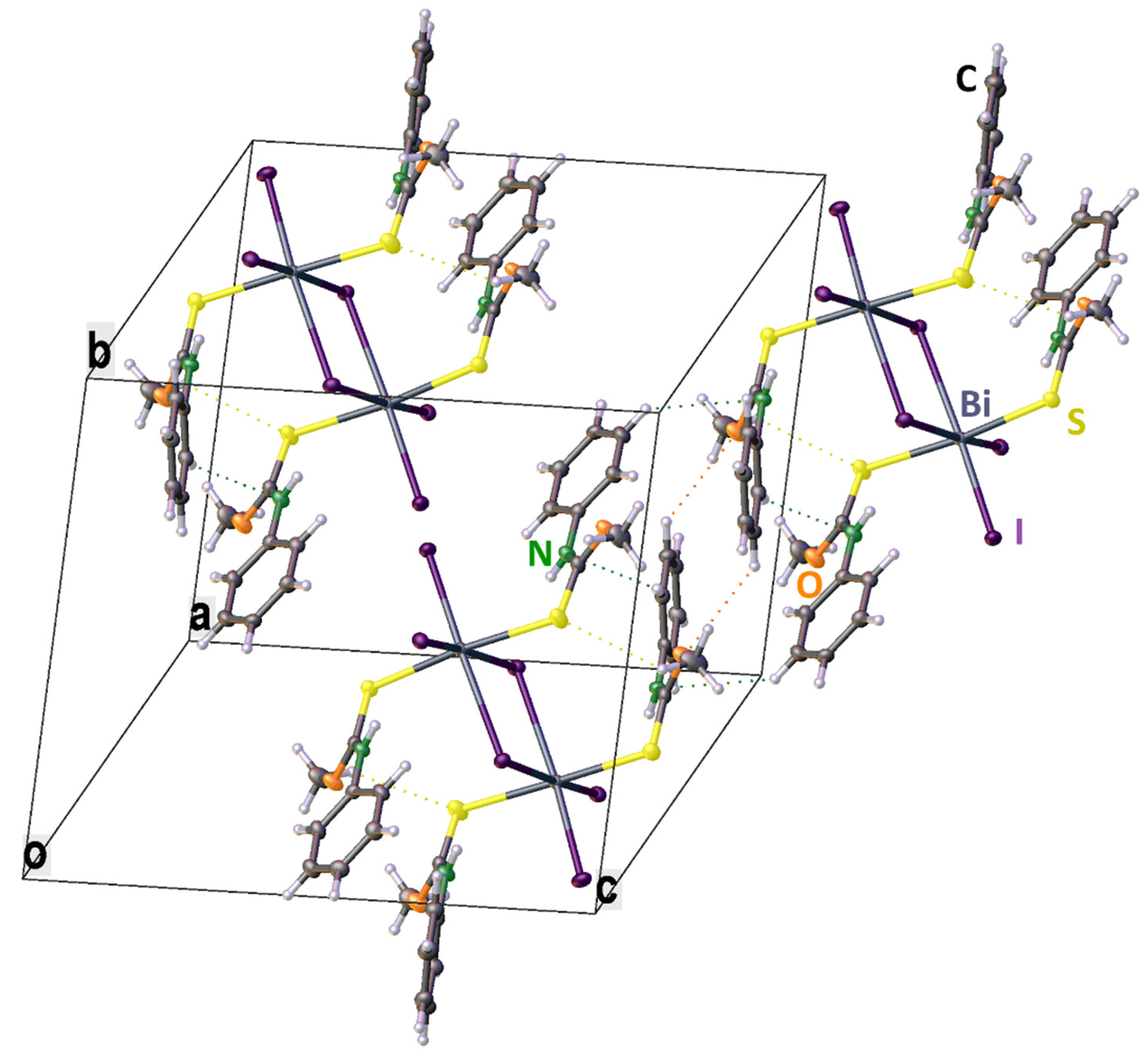
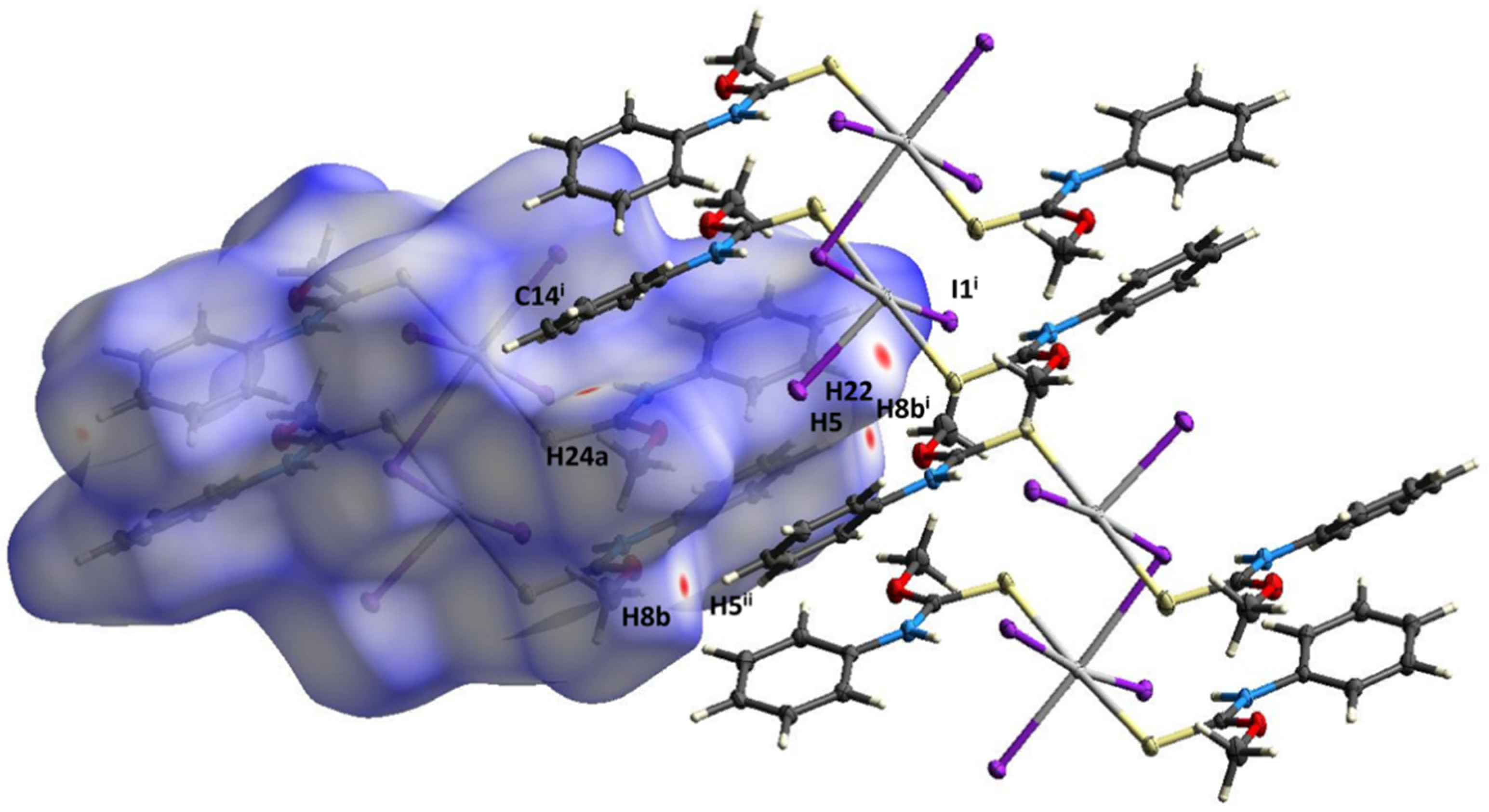
2. Results
3. Discussion
4. Experimental
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Németh, A.G.; Keserű, G.M.; Ábrányi-Balogh, P. A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: A mild, catalyst-free approach towards O-thiocarbamates and dithiocarbamates. Beilstein J. Org. Chem. 2019, 15, 1523–1533. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, Q.; Yang, F.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Sulfur Conversion to Multifunctional Poly(O-thiocarbamate)s through Multicomponent Polymerizations of Sulfur, Diols, and Diisocyanides. J. Am. Chem. Soc. 2021, 143, 3944–3950. [Google Scholar] [CrossRef]
- Shadap, L.; Tyagi, J.L.; Poluri, K.M.; Novikov, S.; Timothy Lo, C.W.; Mozharivskyj, Y.; Kollipara, M.R. Insights to the strained thiocarbamate derivative complexes of platinum group metals induced by azide as a co-ligand: Characterization and biological studies. J. Organomet. Chem. 2020, 920, 121345. [Google Scholar] [CrossRef]
- Krátky, M.; Volková, M.; Novotná, E.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. Synthesis and biological activity of new salicylanilideN,N-disubstituted carbamates and thiocarbamates. Bioorg. Med. Chem. 2014, 22, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Le, C.; Yeung, Y.Y. Enantioselective bromolactonization of cis-1,2-disubstituted olefinic acids using an amino-thiocarbamate catalyst. Chem. Commun. 2012, 48, 5793–5795. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Edu. 1987, 64, 561–567. [Google Scholar] [CrossRef]
- Yeo, C.I.; Halim, S.N.A.; Weng Ng, S.; Tan, S.L.; Zukerman-Schpector, J.; Ferreira, M.A.B.; Tiekink, E.R.T. Investigation of putative arene-C–H···π (quasi-chelatering) interactions in copper(I) crystal structures. Chem. Commun. 2014, 50, 5984–5986. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Clemente, D.A.; Sindellari, L.; Tondello, E. Preparation, properties, and crystal structure of dichlorobis-(O-ethyl thiocarbamate) mercury(II). J. Chem. Soc. Dalton Trans. 1975, 5, 449–452. [Google Scholar] [CrossRef]
- Bardi, R.; Piazzesi, A.M.; Del, P.; Trincia, L. Platinum(II) halide complexes with thiocarbamic esters. Structure of trans-bis[O-ethyl(N-ethyl)thiocarbamate]diiodoplatinum(II), [Pt(ETC)2I2]. Acta Cryst. 1987, C43, 1281–1284. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Sirinkaya, E.T.; Cakmak, M.; Gürgan, M.; Ceyhan, D.; Panagiotou, N.; Tasiopoulos, A.J. Structural and biological features of bismuth(III) halide complexes with heterocyclic thioamides. J. Mol. Struct. 2021, 1227, 129730. [Google Scholar] [CrossRef]
- Battaglia, L.P.; Corradi, A.B. Synthesis and X-ray crystal structure of trichlorotris[1-phenyl-3-(2-pyridyl)-2-thiourea-S]bismuth(III) and di-µ-chloro-tetrachlorotetrakis (NN′-diethylimidazolidine-2-thione-S) dibismuth(III). J. Chem. Soc. Dalton Trans. 1983, 11, 2425–2428. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Hadjikakou, N.; Tasiopoulos, A.J. Structural architectures and biological properties of main group bismuth(III) iodide complexes with heterocyclic thioamides. Inorg. Him. Acta. 2019, 497, 119094. [Google Scholar] [CrossRef]
- Arar, W.; Khatyr, A.; Knorr, M.; Brieger, L.; Krupp, A.; Strohmann, C.; Efrit, M.L.; Ben Akacha, A. Synthesis, crystal Structures and hirshfeld analyses of phosphonothioamidates (EtO)2P(=O)C(=S)N(H)R (R = Cy, Bz) and their coordination on CuI and HgX2 (X = Br, I). Phosphorus Sulfur. Silicon. Relat. Elem. 2021, 196, 845–858. [Google Scholar] [CrossRef]
- Hameau, A.; Guyon, F.; Knorr, M.; Enescu, M.; Strohmann, C. Self-Assembly of Dithiolene-based Coordination Polymers of Mercury(II): Dithioether versus Thiocarbonyl Bonding. Monatsh. Chem. 2006, 137, 545–555. [Google Scholar] [CrossRef]
- Guyon, F.; Hameau, A.; Khatyr, A.; Knorr, M.; Amrouche, H.; Fortin, D.; Harvey, P.D.; Strohmann, C.; Ndiaye, A.L.; Huch, V.; et al. Syntheses, Structures, and Photophysical Properties of Mono- and Dinuclear Sulfur-Rich Gold(I) Complexes. Inorg. Chem. 2008, 47, 7483–7492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hameau, A.; Guyon, F.; Khatyr, A.; Knorr, M.; Strohmann, C. 4,5-Bis(methylthio)-1,3-dithiole-2-thione, a versatile sulphur-rich building block for the self-assembly of Cu(I) and Ag(I) coordination polymers: Dithioether versus thiocarbonyl bonding. Inorg. Chim. Acta 2012, 388, 60–70. [Google Scholar] [CrossRef]
- Chian Sing Lai, S.Y.H.; Tiekink, E.R.T. O-Methyl N-phenylthiocarbamate. Acta Cryst. 2003, E59, o1155–o1156. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. University of Western Australia. 2017. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 1 June 2022).
- Battaglia, L.P.; Corradi, A.B. Structural investigations on bismuth–thiourea derivative adducts: Crystal structures of tetra[1-allyl-3-(2-pyridyl)thiourea-S]di-µ-chloro-tetrachlorodibismuth(III) and hexa[1-allyl-3-(2-pyridyl)thiourea-S]bismuth(III) nitrate. J. Chem. Soc. Dalton Trans. 1981, 1, 23–26. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arar, W.; Khatyr, A.; Knorr, M.; Strohmann, C.; Schmidt, A. Bis(μ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth. Molbank 2022, 2022, M1381. https://doi.org/10.3390/M1381
Arar W, Khatyr A, Knorr M, Strohmann C, Schmidt A. Bis(μ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth. Molbank. 2022; 2022(2):M1381. https://doi.org/10.3390/M1381
Chicago/Turabian StyleArar, Wafa, Abderrahim Khatyr, Michael Knorr, Carsten Strohmann, and Annika Schmidt. 2022. "Bis(μ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth" Molbank 2022, no. 2: M1381. https://doi.org/10.3390/M1381
APA StyleArar, W., Khatyr, A., Knorr, M., Strohmann, C., & Schmidt, A. (2022). Bis(μ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth. Molbank, 2022(2), M1381. https://doi.org/10.3390/M1381






