2-((4-Phenyl-5-(2-(p-tolylamino)ethyl)-4H-1,2,4-triazol-3-yl)thio)-N′-(1-phenylethylidene)acetohydrazide
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
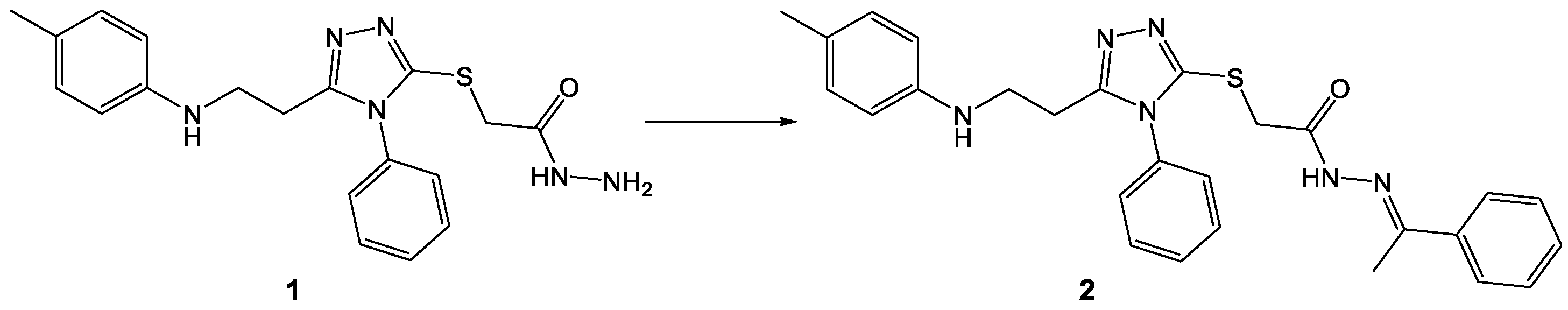
3.1. Synthesis
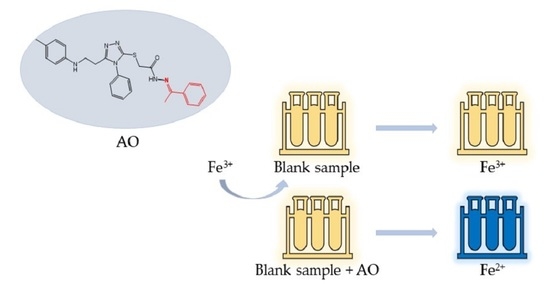
3.2. Evaluation of Antioxidant Activity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.J.; Normile, C.; Irnaten, M.; O’Brien, C. The Intertwined Roles of Oxidative Stress and Endoplasmic Reticulum Stress in Glaucoma. Antioxidants 2022, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Lee, C.-Y.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.-L.; Looi, W.-F.; Huang, S.-H.; Wang, H.; Chan, Y.-H. Oxidative Damage in Parkinson Disease: Measurement Using Accurate Biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent Advances in the Chemistry of 1,2,4-Triazoles: Synthesis, Reactivity and Biological Activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An Insight on Medicinal Attributes of 1,2,4-Triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Beresnevičius, Z.J. The Synthesis of Azole Derivatives from 3-[(4-Methylphenyl)Amino]Propanehydrazide and Its N′-Phenylcarbamoyl Derivatives, and Their Antibacterial Activity. Mon. Chem. 2012, 143, 1441–1450. [Google Scholar] [CrossRef]
- Scarim, C.B.; Pavan, F.R. Thiazole, Triazole, Thio- and Semicarbazone Derivatives—Promising Moieties for Drug Development for the Treatment of Tuberculosis. Eur. J. Med. Chem. Rep. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Sahu, J.K.; Ganguly, S.; Kaushik, A. Triazoles: A Valuable Insight into Recent Developments and Biological Activities. Chin. J. Nat. Med. 2013, 11, 456–465. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial Activity Study of 1,2,4-Triazole Derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Shaker, R.M. The Chemistry of Mercapto- and Thione-Substituted 1,2,4-Triazoles and Their Utility in Heterocyclic Synthesis. Arkivoc 2006, 2006, 59–112. [Google Scholar] [CrossRef] [Green Version]
- Slivka, M.V.; Korol, N.I.; Fizer, M.M. Fused Bicyclic 1,2,4-triazoles with One Extra Sulfur Atom: Synthesis, Properties, and Biological Activity. J. Heterocycl. Chem. 2020, 57, 3236–3254, jhet.4044. [Google Scholar] [CrossRef]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent Advances Bioactive 1,2,4-Triazole-3-Thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef] [PubMed]
- Legru, A.; Verdirosa, F.; Hernandez, J.-F.; Tassone, G.; Sannio, F.; Benvenuti, M.; Conde, P.-A.; Bossis, G.; Thomas, C.A.; Crowder, M.W.; et al. 1,2,4-Triazole-3-Thione Compounds with a 4-Ethyl Alkyl/Aryl Sulfide Substituent Are Broad-Spectrum Metallo-β-Lactamase Inhibitors with Re-Sensitization Activity. Eur. J. Med. Chem. 2021, 226, 113873. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-L.; Zhang, L.-Y.; Zhan, Y.-Z.; Zhang, Y.; Zhang, X.; Wang, L.-Z.; Li, Z.-M. Synthesis and Biological Activities of Novel 1,2,4-Triazole Thiones and Bis(1,2,4-Triazole Thiones) Containing Phenylpyrazole and Piperazine Moieties. J. Fluor. Chem. 2016, 184, 36–44. [Google Scholar] [CrossRef]
- Makuch-Kocka, A.; Andres-Mach, M.; Zagaja, M.; Śmiech, A.; Pizoń, M.; Flieger, J.; Cielecka-Piontek, J.; Plech, T. Effect of Chronic Administration of 5-(3-Chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms. Int. J. Mol. Sci. 2021, 22, 3358. [Google Scholar] [CrossRef]
- Gavara, L.; Verdirosa, F.; Legru, A.; Mercuri, P.S.; Nauton, L.; Sevaille, L.; Feller, G.; Berthomieu, D.; Sannio, F.; Marcoccia, F.; et al. 4-(N-Alkyl- and -Acyl-Amino)-1,2,4-Triazole-3-Thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition. Biomolecules 2020, 10, 1094. [Google Scholar] [CrossRef]
- Patel, K.R.; Brahmbhatt, J.G.; Pandya, P.A.; Daraji, D.G.; Patel, H.D.; Rawal, R.M.; Baran, S.K. Design, Synthesis and Biological Evaluation of Novel 5-(4-Chlorophenyl)-4-Phenyl-4H-1,2,4-Triazole-3-Thiols as an Anticancer Agent. J. Mol. Struct. 2021, 1231, 130000. [Google Scholar] [CrossRef]
- Aly, A.A.; Hassan, A.A.; Makhlouf, M.M.; Bräse, S. Chemistry and Biological Activities of 1,2,4-Triazolethiones—Antiviral and Anti-Infective Drugs. Molecules 2020, 25, 3036. [Google Scholar] [CrossRef]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Di Meo, F.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.-F.F.; et al. Antioxidant Properties of Phenolic Schiff Bases: Structure–Activity Relationship and Mechanism of Action. J. Comput. Aided Mol. Des. 2013, 27, 951–964. [Google Scholar] [CrossRef]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Beresnevičius, Z.J. The Synthesis of S-Substituted Derivatives of 3-[2-[(4-Methylphenyl)Amino]Ethyl]-4-Phenyl-4,5-Dihydro-1H-1,2,4-Triazole-5-Thiones and Their Antioxidative Activity. Mon. Chem. 2014, 145, 319–327. [Google Scholar] [CrossRef]
- Tumosienė, I.; Kantminienė, K.; Jonuškienė, I.; Peleckis, A.; Belyakov, S.; Mickevičius, V. Synthesis of 1-(5-Chloro-2-Hydroxyphenyl)-5-Oxopyrrolidine-3-Carboxylic Acid Derivatives and Their Antioxidant Activity. Molecules 2019, 24, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumosienė, I.; Kantminienė, K.; Klevinskas, A.; Petrikaitė, V.; Jonuškienė, I.; Mickevičius, V. Antioxidant and Anticancer Activity of Novel Derivatives of 3-[(4-Methoxyphenyl)Amino]Propanehydrazide. Molecules 2020, 25, 2980. [Google Scholar] [CrossRef] [PubMed]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Mickevičius, V.; Petrikaitė, V. Novel N-Substituted Amino Acid Hydrazone-Isatin Derivatives: Synthesis, Antioxidant Activity, and Anticancer Activity in 2D and 3D Models In Vitro. Int. J. Mol. Sci. 2021, 22, 7799. [Google Scholar] [CrossRef] [PubMed]
- Parašotas, I.; Urbonavičiūtė, E.; Anusevičius, K.; Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Vaickelionienė, R.; Mickevičius, V. Synthesis and Biological Evaluation of Novel Di- and Trisubstituted Thiazole Derivatives. Heterocycles 2017, 94, 1074. [Google Scholar] [CrossRef]
- Tumosienė, I.; Peleckis, A.; Jonuškienė, I.; Vaickelionienė, R.; Kantminienė, K.; Šiugždaitė, J.; Beresnevičius, Z.J.; Mickevičius, V. Synthesis of Novel 1,2- and 2-Substituted Benzimidazoles with High Antibacterial and Antioxidant Activity. Mon. Chem. 2018, 149, 577–594. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šermukšnytė, A.; Jonuškienė, I.; Kantminienė, K.; Beresnevičius, Z.J.; Tumosienė, I. 2-((4-Phenyl-5-(2-(p-tolylamino)ethyl)-4H-1,2,4-triazol-3-yl)thio)-N′-(1-phenylethylidene)acetohydrazide. Molbank 2022, 2022, M1380. https://doi.org/10.3390/M1380
Šermukšnytė A, Jonuškienė I, Kantminienė K, Beresnevičius ZJ, Tumosienė I. 2-((4-Phenyl-5-(2-(p-tolylamino)ethyl)-4H-1,2,4-triazol-3-yl)thio)-N′-(1-phenylethylidene)acetohydrazide. Molbank. 2022; 2022(2):M1380. https://doi.org/10.3390/M1380
Chicago/Turabian StyleŠermukšnytė, Aida, Ilona Jonuškienė, Kristina Kantminienė, Zigmuntas Jonas Beresnevičius, and Ingrida Tumosienė. 2022. "2-((4-Phenyl-5-(2-(p-tolylamino)ethyl)-4H-1,2,4-triazol-3-yl)thio)-N′-(1-phenylethylidene)acetohydrazide" Molbank 2022, no. 2: M1380. https://doi.org/10.3390/M1380
APA StyleŠermukšnytė, A., Jonuškienė, I., Kantminienė, K., Beresnevičius, Z. J., & Tumosienė, I. (2022). 2-((4-Phenyl-5-(2-(p-tolylamino)ethyl)-4H-1,2,4-triazol-3-yl)thio)-N′-(1-phenylethylidene)acetohydrazide. Molbank, 2022(2), M1380. https://doi.org/10.3390/M1380