Modification of 1-Hexene Vinylidene Dimer into Primary and Tertiary Alkanethiols
Abstract
1. Introduction
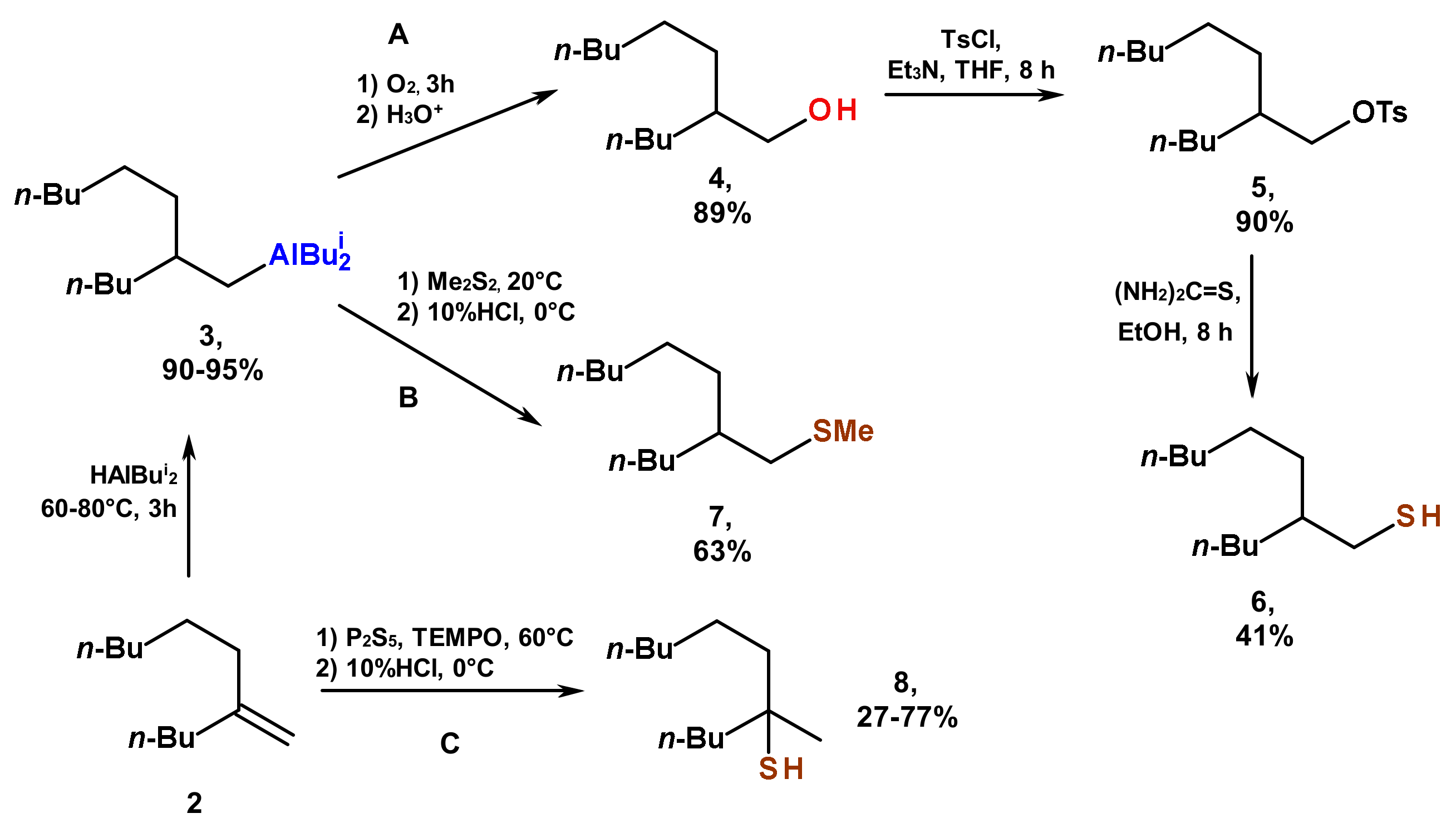
2. Results and Discussion
3. Materials and Methods
General Procedures
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pronin, V.A.; Usol’tseva, M.V.; Trofimov, B.A.; Vyalykh, E.P.; Shastina, Z.N. Extractive power of certain mercaptans with respect to Au (III), Ag (I), Pt (IV), and Pd (II). J. Appl. Chem. USSR 1973, 46, 2758–2760. [Google Scholar]
- Chen, D.; Cui, P.; Cao, H.; Yang, J. A 1-dodecanethiol-based phase transfer protocol for the highly efficient extraction of noble metal ions from aqueous phase. J. Environ. Sci. 2015, 29, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Holmlin, R.E.; Haag, R.; Chabinyc, M.L.; Ismagilov, R.F.; Cohen, A.E.; Terfort, A.; Rampi, M.A.; Whitesides, G.M. Electron Transport through Thin Organic Films in Metal−Insulator−Metal Junctions Based on Self-Assembled Monolayers. J. Am. Chem. Soc. 2001, 123, 5075–5085. [Google Scholar] [CrossRef] [PubMed]
- Urcuyo, R.; Cortés, E.; Rubert, A.A.; Benitez, G.; Montero, M.L.; Tognalli, N.G.; Fainstein, A.; Vela, M.E.; Salvarezza, R.C. Aromatic and Aliphatic Thiol Self-Assembled Monolayers on Au: Anchoring and Delivering Copper Specie. J. Phys. Chem. C 2011, 115, 24707–24717. [Google Scholar] [CrossRef]
- Denayer, J.; Delhalle, J.; Mekhalif, Z. Comparative study of copper surface treatment with self-assembled monolayers of aliphatic thiol, dithiol and dithiocarboxylic acid. J. Electroanal. Chem. 2009, 637, 43–49. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Chen, T.; Xie, J. Mechanistic exploration and controlled synthesis of precise thiolate-gold Nanoclusters. Coord. Chem. Rev. 2016, 329, 1–15. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Rambukwella, M.; Sakthivel, N.A.; Delcamp, J.H.; Sementa, L.; Fortunelli, A.; Dass, A. Ligand Structure Determines Nanoparticles’ Atomic Structure, Metal-Ligand Interface and Properties. Front. Chem. 2018, 6, 330. [Google Scholar] [CrossRef]
- Wardell, J.L. Preparation of thiols. In The Thiol Group; Patai, S., Ed.; John Wiley & Sons Ltd: Bristol, UK, 1974. [Google Scholar] [CrossRef]
- Hoyle, C.; Bowman, C. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene ‘‘click’’ reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Snow, A.W.; Foos, E.E. Conversion of Alcohols to Thiols via Tosylate Intermediates. Synthesis 2003, 4, 509–512. [Google Scholar] [CrossRef][Green Version]
- Maurya, C.K.; Gupta, P.K. Phosphorus Pentasulfide Mediated Conversion of Primary Carbamates into Thiols. Synlett 2017, 28, 1649–1651. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta Catalysis in a-Olefin Transformations: Reaction Mechanisms and Product Design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-Catalyzed Dimerization of 1-Hexene: Two-stage Activation and Structure–Catalytic Performance Relationship. Cat. Comm. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally Uniform 1-Hexene, 1-Octene, and 1-Decene Oligomers: Zirconocene/MAO-Catalyzed Preparation, Characterization, and Prospects of Their Use as low-viscosity Low-temperature Oil Base Stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers 2020, 12, 1590. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules 2020, 25, 2216. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization. Catalysts 2021, 11, 39. [Google Scholar] [CrossRef]
- Kovyazin, P.V.; Bikmeeva, A.K.; Islamov, D.N.; Yanybin, V.M.; Tyumkina, T.V.; Parfenova, L.V. Ti subgroup metallocene-catalyzed synthesis of 1-hexene dimers and tetramers. Molecules 2021, 26, 2775. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G.; Zolotarev, A.P.; Tolstikov, G.A. Nontraditional approach to the synthesis of 3-substituted tetrahydrothiophenes and tetrahydroselenophenes. Russ. Chem. Bull. 1989, 38, 1324. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G.; Azhgaliev, M.N.; Muslukhov, R.R. Synthesis and transformations of metallocycles. Russ. Chem. Bull. 1994, 43, 255–257. [Google Scholar] [CrossRef]

| Entry | Substrate | OAC | Solvent | Thiolation Reagent | Substrate:HAlBu i2:Reagent | t, °C | Time, h | Product, Yield |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | toluene | P2S5 | 1:0:1 | 60 | 16 | 6, <3% | |
| 2 | 5 | EtOH | (H2N)2C=S | 1:0:1 | 60 | 8 | 6, 41% | |
| 3 | 2 | HAlBui2 | toluene | (H2N)2C=S | 1:1:(1–3) | 80 | 16 | |
| 4 | 2 | HAlBui2 | toluene | P2S5 | 1:1:(1–3) | 60 | 16 | 6, <5% |
| 5 | 2 | HAlBui2 | toluene | Me2S2 | 1:1:(1–3) | 20 | 16 | 7, 63% |
| 6 | 2 | toluene | P2S5 | 1:0:1 | 80 | 16 | ||
| 7 | 2 | toluene | P2S5 a | 1:0:1 | 60 | 16 | 8, 77% | |
| 8 | 2 | toluene | P2S5 a | 1:0:0.5 | 60 | 16 | 8, 40% | |
| 9 | 2 | hexane | P2S5 a | 1:0:1 | 60 | 16 | 8, 37% | |
| 10 | 2 | CHCl3 | P2S5 a | 1:0:1 | 60 | 16 | 8, 29% | |
| 11 | 2 | CH2Cl2 | P2S5 a | 1:0:1 | 20 | 16 | 8, 41% | |
| 12 | 2 | Et2O | P2S5 a | 1:0:1 | 20 | 16 | 8, 33% | |
| 13 | 2 | THF b | P2S5 a | 1:0:1 | 60 | 16 | 8, 27% b | |
| 14 | 2 | H2O | P2S5 a | 1:0:1 | 60 | 16 | 8, 30% | |
| 15 | 2 | 10% HCl | P2S5 a | 1:0:1 | 60 | 16 | 8, 54% | |
| 16 | 2 | EtOH | P2S5 a | 1:0:1 | 60 | 16 | 8, 9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R.; Parfenova, L.V. Modification of 1-Hexene Vinylidene Dimer into Primary and Tertiary Alkanethiols. Molbank 2022, 2022, M1379. https://doi.org/10.3390/M1379
Kovyazin PV, Bikmeeva AK, Palatov ER, Parfenova LV. Modification of 1-Hexene Vinylidene Dimer into Primary and Tertiary Alkanethiols. Molbank. 2022; 2022(2):M1379. https://doi.org/10.3390/M1379
Chicago/Turabian StyleKovyazin, Pavel V., Almira Kh. Bikmeeva, Eldar R. Palatov, and Lyudmila V. Parfenova. 2022. "Modification of 1-Hexene Vinylidene Dimer into Primary and Tertiary Alkanethiols" Molbank 2022, no. 2: M1379. https://doi.org/10.3390/M1379
APA StyleKovyazin, P. V., Bikmeeva, A. K., Palatov, E. R., & Parfenova, L. V. (2022). Modification of 1-Hexene Vinylidene Dimer into Primary and Tertiary Alkanethiols. Molbank, 2022(2), M1379. https://doi.org/10.3390/M1379







