Ethyl (E)-(3-(4-((4-bromobenzyl)oxy)phenyl)acryloyl)glycinate
Abstract
:1. Introduction
2. Results and Discussion
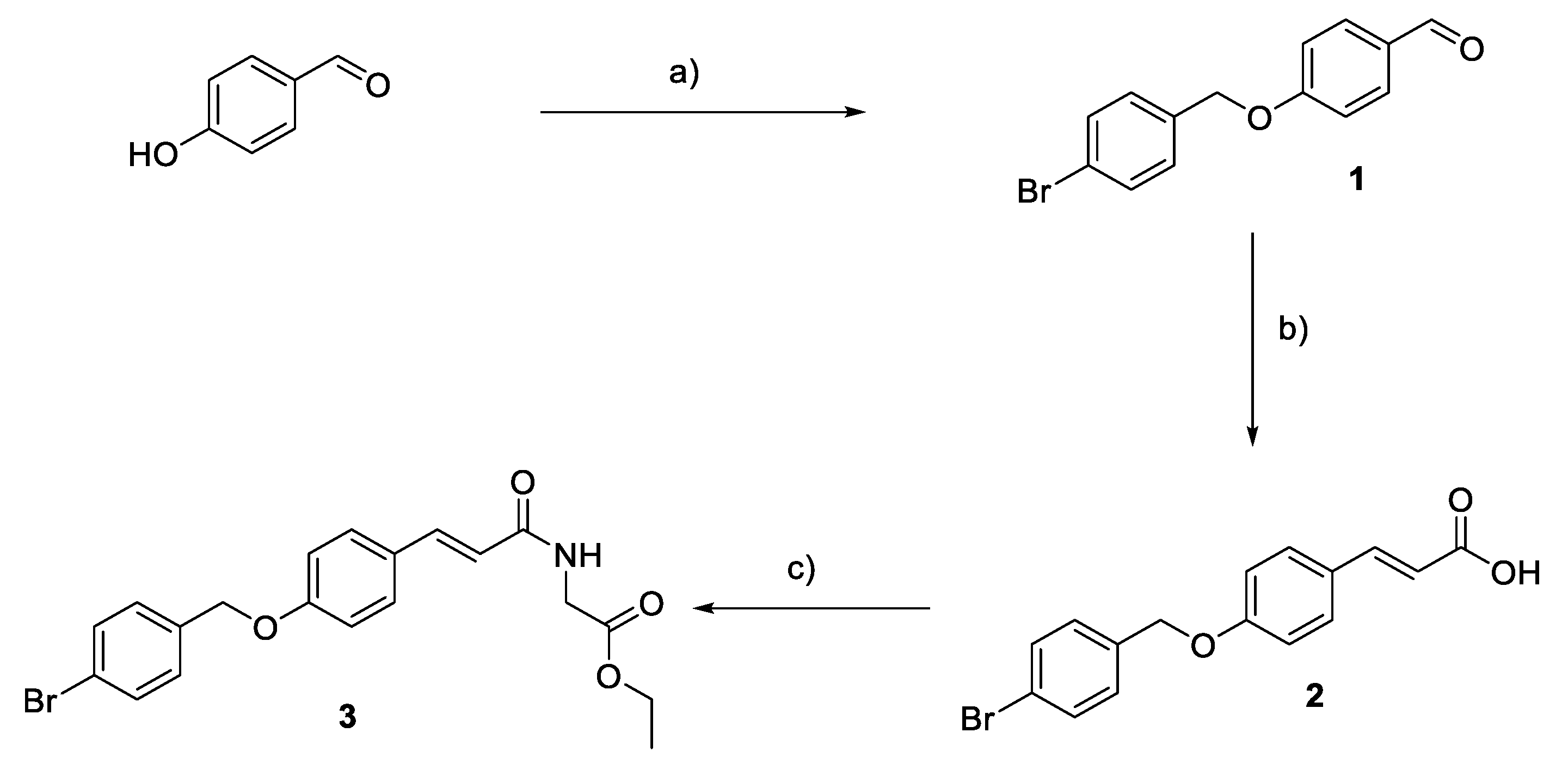
2.1. Chemistry
2.2. Drug-Likeness Studies
2.3. Biological Evaluation
3. Materials and Methods
3.1. General Information
3.2. Chemistry General Procedure
3.2.1. Synthesis of 4-(Bromobenzyl)oxy-benzaldehyde (1)
3.2.2. Synthesis of (E)-3-(4-((4-bromobenzyl)oxy)phenyl)acrylic Acid (2)
3.2.3. Synthesis of Ethyl (E)-(3-(4-((4-bromobenzyl)oxy)phenyl)acryloyl)glycinate (3)
3.3. Biological In Vitro Assays
Inhibition of Ovine Cyclooxygenase-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, T.V.; Hoang, H.N.T.; Nguyen, H.T.; Nguyen, H.M.; Huynh, C.T.; Vu, T.Y.; Do, A.T.; Nguyen, N.H.; Do, B.H. Anti-Inflammatory and Antimicrobial Activities of Compounds Isolated from Distichochlamys benenica. BioMed Res. Int. 2021, 2021, 6624347. [Google Scholar] [CrossRef] [PubMed]
- Ambati, G.G.; Jachak, S.M. Natural Product Inhibitors of Cyclooxygenase (COX) Enzyme: A Review on Current Status and Future Perspectives. Curr. Med. Chem. 2021, 28, 1877–1905. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioffi, C.L. Modulation of Glycine-Mediated Spinal Neurotransmission for the Treatment of Chronic Pain. J. Med. Chem. 2018, 61, 2652–2679. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, I.; Pontiki, E.; Hadjipavlou-Litina, D. Targeting Inflammation with Conjugated Cinnamic Amides, Ethers and Esters. Lett. Drug Des. Discov. 2020, 17, 3–11. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis of Phenyl-substituted Amides with Antioxidant and Antiinflammatory activity as Novel Lipoxygenase Inhibitors. Med. Chem. 2007, 3, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, D.; Du, H.; Zheng, C.; Li, J.; Piao, H.; Li, J.; Sun, L. Synthesis and biological evaluation of tryptophan-derived rhodanine derivatives as PTP1B inhibitors and anti-bacterial agents. Eur. J. Med. Chem. 2019, 172, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lutjen, A.B.; Quirk, M.A.; Barbera, A.M.; Kolonko, E.M. Synthesis of (E)-cinnamyl ester derivatives via a greener Steglich esterification. Bioorganic Med. Chem. 2018, 26, 5291–5298. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Fernandes, J.; Gattass, C.R. Topological Polar Surface Area Defines Substrate Transport by Multidrug Resistance Associated Protein 1 (MRP1/ABCC1). J. Med. Chem. 2009, 52, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Siskos, A.; Pontiki, E.; Hadjipavlou-Litina, D. Hybridization of Curcumin Analogues with Cinnamic Acid Derivatives as Multi-Target Agents Against Alzheimer’s Disease Targets. Molecules 2020, 25, 4958. [Google Scholar] [CrossRef] [PubMed]
| milogP a | TPSA b | No. of atoms | No of O and N c | No of OH and NH d | No of Violations | No of Rotational Bonds e | Volume f | MW g | BBB score h |
|---|---|---|---|---|---|---|---|---|---|
| 4.47 | 64.64 | 26 | 5 | 1 | 0 | 9 | 336.06 | 418.29 | 4.21 |
| Compound | Structure | COX Inhibition (IC50) μM |
|---|---|---|
| 2 | 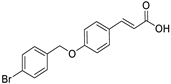 | no |
| 3 | 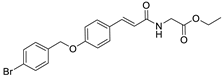 | 6 μΜ |
| Indomethacin # | 1.12 μΜ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotopoulos, I.; Papaioannou, G.-C.; Hadjipavlou-Litina, D. Ethyl (E)-(3-(4-((4-bromobenzyl)oxy)phenyl)acryloyl)glycinate. Molbank 2022, 2022, M1378. https://doi.org/10.3390/M1378
Fotopoulos I, Papaioannou G-C, Hadjipavlou-Litina D. Ethyl (E)-(3-(4-((4-bromobenzyl)oxy)phenyl)acryloyl)glycinate. Molbank. 2022; 2022(2):M1378. https://doi.org/10.3390/M1378
Chicago/Turabian StyleFotopoulos, Ioannis, George-Christos Papaioannou, and Dimitra Hadjipavlou-Litina. 2022. "Ethyl (E)-(3-(4-((4-bromobenzyl)oxy)phenyl)acryloyl)glycinate" Molbank 2022, no. 2: M1378. https://doi.org/10.3390/M1378
APA StyleFotopoulos, I., Papaioannou, G.-C., & Hadjipavlou-Litina, D. (2022). Ethyl (E)-(3-(4-((4-bromobenzyl)oxy)phenyl)acryloyl)glycinate. Molbank, 2022(2), M1378. https://doi.org/10.3390/M1378








