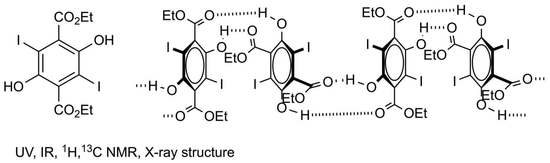
Diethyl 2,5-Dihydroxy-3,6-diiodoterephthalate
Abstract
:1. Introduction
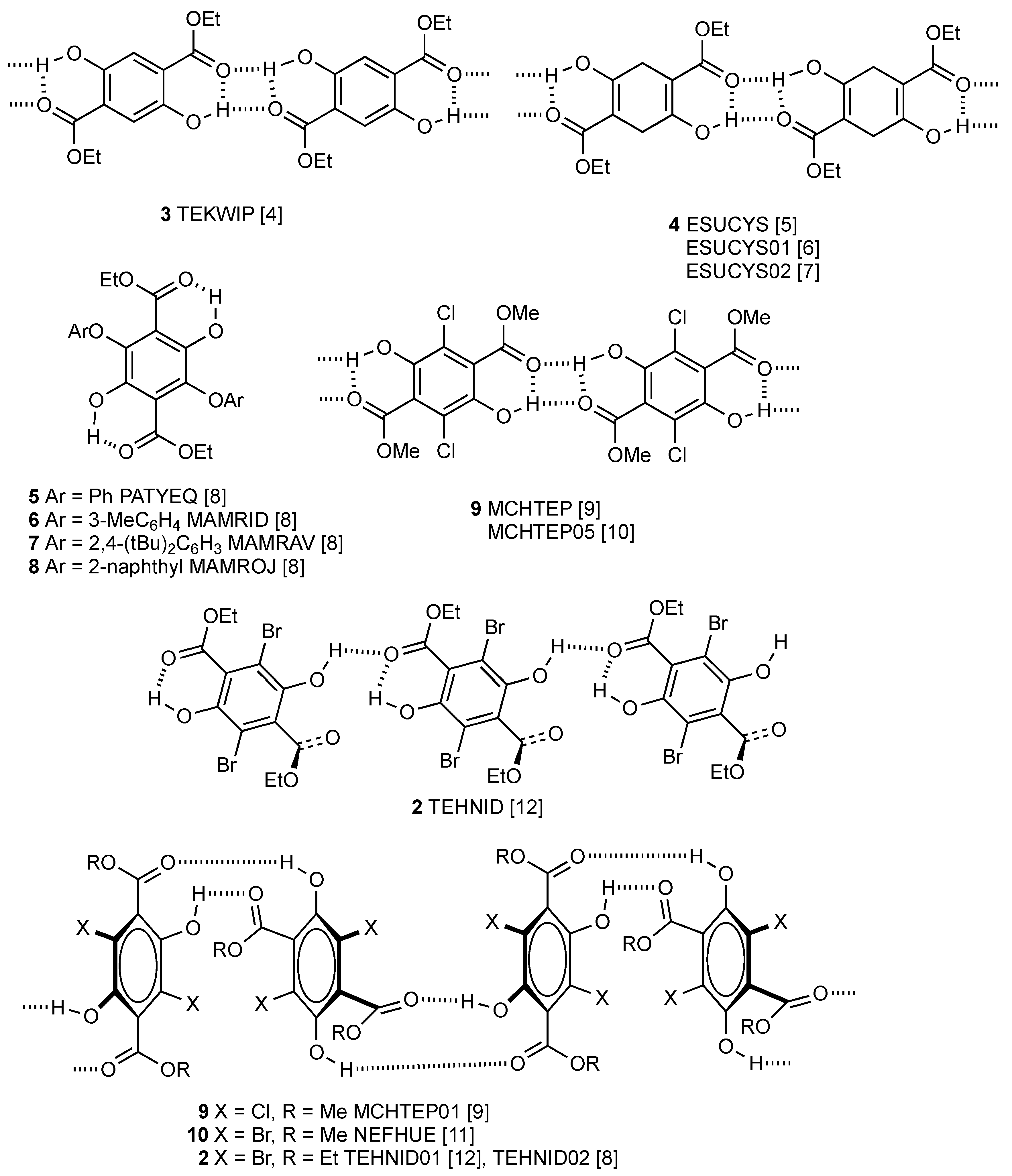
2. Results
3. Experimental Section
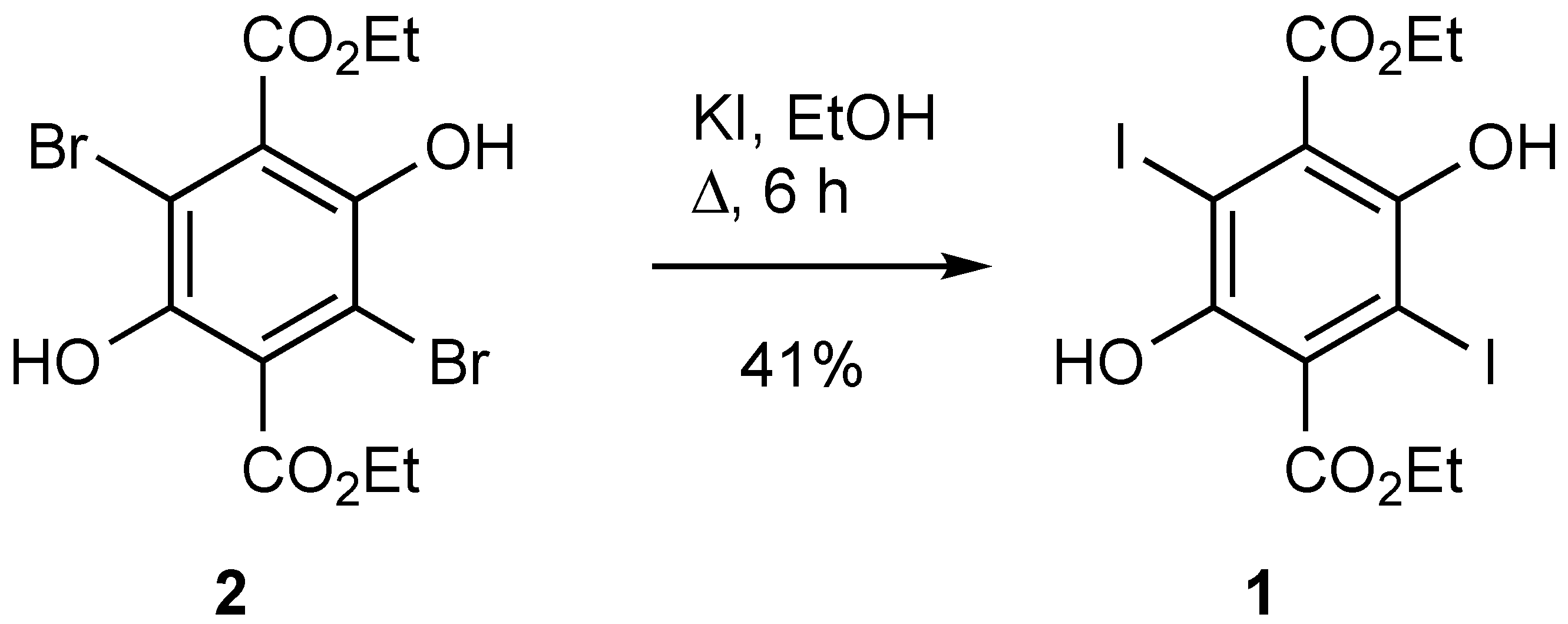
Diethyl 2,5-Dihydroxy-3,6-Diiodoterephthalate (1)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guinchard, J. Ueber einige Derivate des Succinylobernsteinsäreesters. Ber. Dtsch. Chem. Ges. 1899, 32, 1742–1744. [Google Scholar] [CrossRef] [Green Version]
- Böniger, M. Ueber desmotrope Derivate des Succinylobernsteinsäureäthers. Ber. Dtsch. Chem. Ges. 1888, 21, 1758–1765. [Google Scholar] [CrossRef] [Green Version]
- Hantzsch, A. Die Chromoisomerie der p-Dioxy-terephthalsäure-Derivate als Phenol-Enol-Isomerie. Ber. Dtsch. Chem. Ges. 1915, 48, 797–816. [Google Scholar] [CrossRef] [Green Version]
- Näther, C.; Bock, H.; Seitz, W.; Nagel, N. Diethyl 2,5-dihydroxyterephthalate at 200K. Acta Crystallogr. Sect. C 1996, 52, 2631–2633. [Google Scholar] [CrossRef]
- Mez, H.-C.; Rihs, G. Chemistry of succinylsuccinic acid derivatives. Part II The crystal and molecular structure of diethyl succinylsuccinate. Helv. Chim. Acta 1973, 56, 2766–2772. [Google Scholar] [CrossRef]
- Frischmann, L.; Langhals, H.; Polborn, K. CCDC 263834: Experimental Crystal Structure Determination; The Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Chandrashaker, V.; Ptaszek, M.; Taniguchi, M.; Lindsey, J.S. Synthesis of diverse acyclic precursors to pyrroles for studies of prebiotic routes to tetrapyrrole macrocycles. New J. Chem. 2016, 40, 8786–8808. [Google Scholar] [CrossRef]
- Benniston, A.C.; Winstanley, T.P.L.; Lemmetyinen, H.; Tkachenko, N.V.; Harrington, R.W.; Wills, C. Large Stokes shift fluorescent dyes based on a highly substituted terephthalic acid core. Org. Lett. 2012, 14, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Byrn, S.R.; Curtin, D.Y.; Paul, I.C. The X-ray crystal structures of the yellow and white forms of dimethyl 3,6-dichloro-2,5-dihydroxyterephthalate and a study of the conversion of the yellow form to the white form in the solid state. J. Am. Chem. Soc. 1972, 94, 890–898. [Google Scholar] [CrossRef]
- Yang, Q.C.; Richardson, M.F.; Dunitz, J.D. Internal molecular motion as forerunner of a phase change involving conformational isomerization. J. Am. Chem. Soc. 1985, 107, 5535–5537. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Abrahams, B.F.; Robson, R. CCDC 1567624: Experimental Crystal Structure Determination; The Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Näther, C.; Nagel, N.; Bock, H.; Seitz, W.; Havlas, Z. Structural, kinetic and thermodynamic aspects of the conformational dimorphism of diethyl 3,6-dibromo-2,5-dihydroxyterephthalate. Acta Crystallogr. Sect. B 1996, 52, 697–706. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

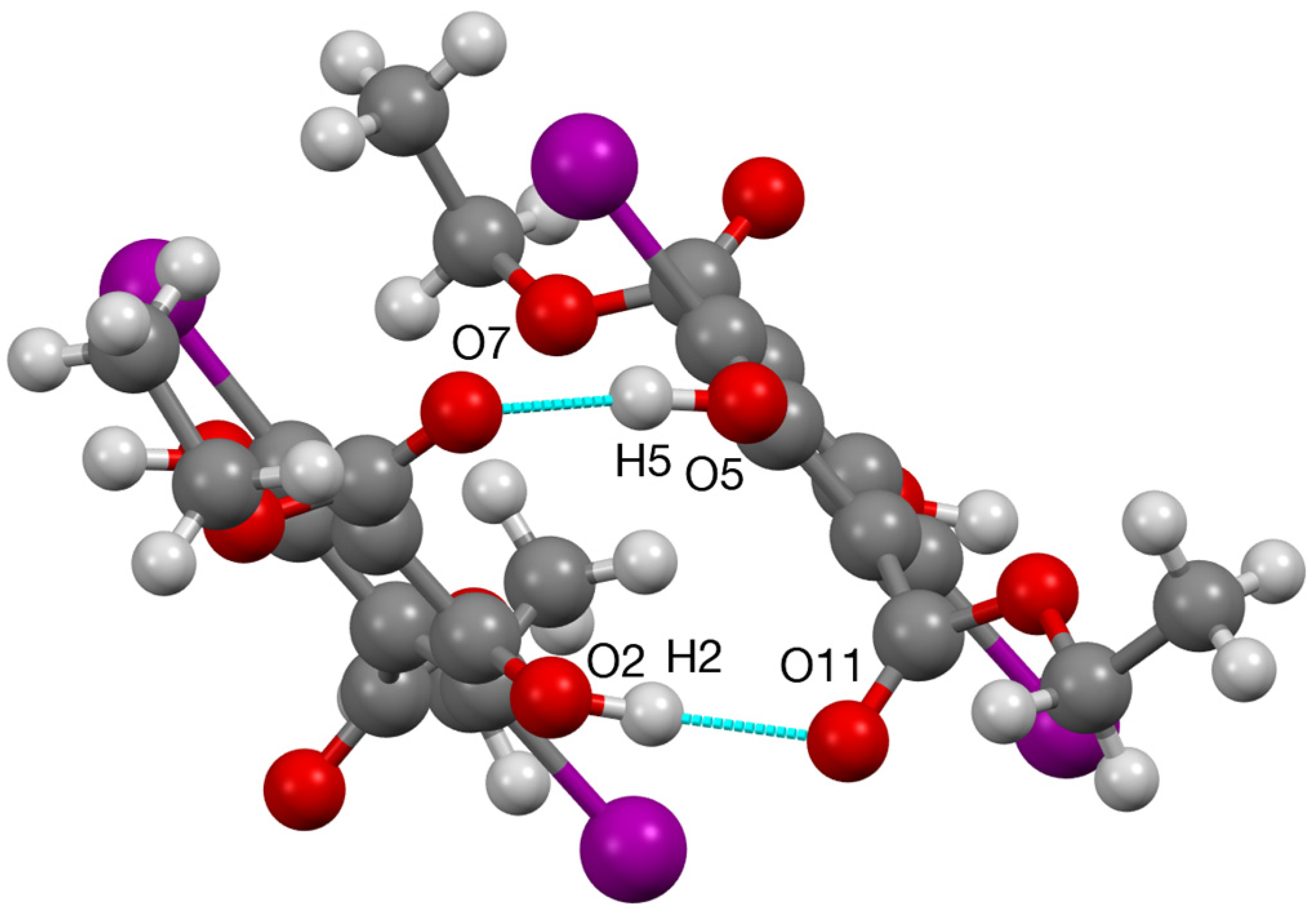

| D—H…A | D—H | H…A | D…A | D—H…A |
|---|---|---|---|---|
| O(2)–H(2)…O(11) | 0.98(3) | 1.90(3) | 2.778(4) | 149(4) |
| O(5)–H(5)…O(7) | 0.98(2) | 1.79(2) | 2.746(3) | 163(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Schindler, N.; Slawin, A.M.Z. Diethyl 2,5-Dihydroxy-3,6-diiodoterephthalate. Molbank 2022, 2022, M1369. https://doi.org/10.3390/M1369
Aitken RA, Schindler N, Slawin AMZ. Diethyl 2,5-Dihydroxy-3,6-diiodoterephthalate. Molbank. 2022; 2022(2):M1369. https://doi.org/10.3390/M1369
Chicago/Turabian StyleAitken, R. Alan, Niti Schindler, and Alexandra M. Z. Slawin. 2022. "Diethyl 2,5-Dihydroxy-3,6-diiodoterephthalate" Molbank 2022, no. 2: M1369. https://doi.org/10.3390/M1369
APA StyleAitken, R. A., Schindler, N., & Slawin, A. M. Z. (2022). Diethyl 2,5-Dihydroxy-3,6-diiodoterephthalate. Molbank, 2022(2), M1369. https://doi.org/10.3390/M1369