(E)-4-(2-(7-Bromo-[1,2,5]thiadiazolo[3,4-c]pyridin-4-yl)vinyl)-N,N-diphenylaniline
Abstract
:1. Introduction
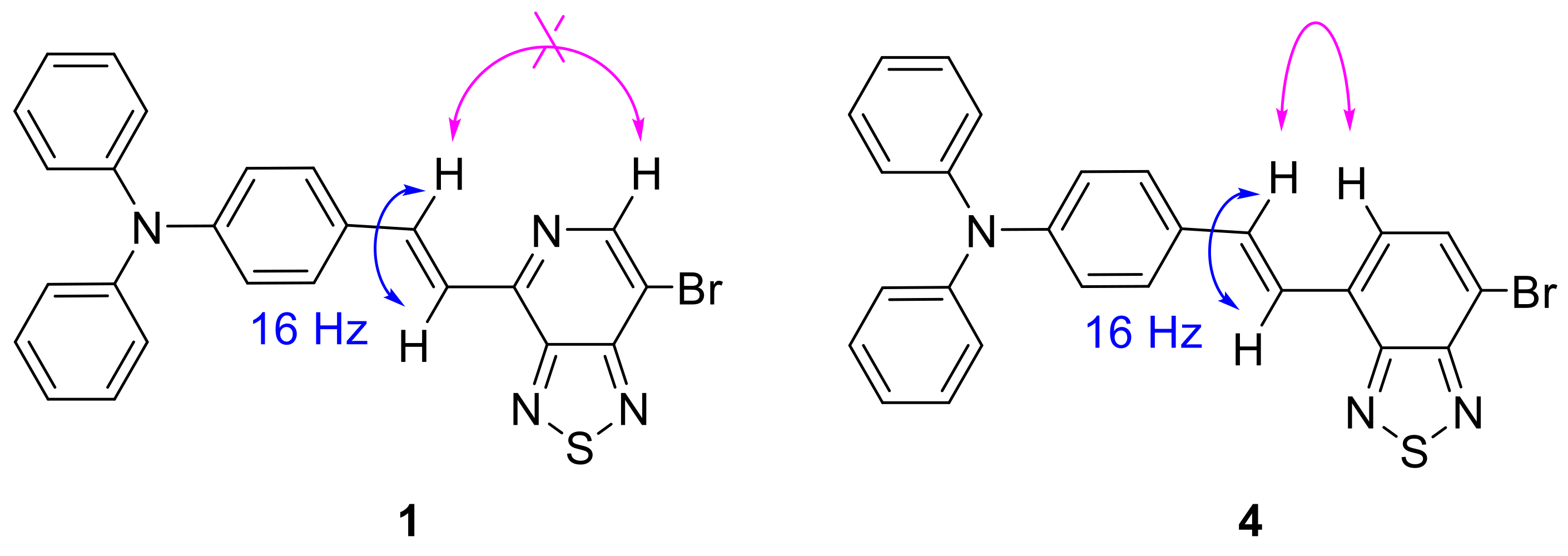
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rakitin, O.A. Recent Developments in the Synthesis of 1,2,5-Thiadiazoles and 2,1,3-Benzothiadiazoles. Synthesis 2019, 51, 4338–4347. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Kato, S.; Matsumoto, T.; Ishi-i, T.; Thiemann, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Yamashita, Y.; Mataka, S. Strongly red-fluorescent novel donor–π-bridge–acceptor–π-bridge–donor (D–π–A–π–D) type 2,1,3-benzothiadiazoles with enhanced two-photon absorption cross-sections. Chem. Commun. 2004, 2342–2343. [Google Scholar] [CrossRef]
- Kato, S.; Matsumoto, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Ishi-i, T.; Mataka, S. Novel 2,1,3-Benzothiadiazole-Based Red-Fluorescent Dyes with Enhanced Two-Photon Absorption Cross-Sections. Chem. -A Eur. J. 2006, 12, 2303–2317. [Google Scholar] [CrossRef]
- He, C.; He, Q.; Yi, Y.; Wu, G.; Bai, F.; Shuai, Z.; Li, Y. Improving the efficiency of solution processable organic photovoltaic devices by a star-shaped molecular geometry. J. Mater. Chem. 2008, 18, 4085. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Zhou, Y.; Pei, J. Surface Modification of Self-Assembled One-Dimensional Organic Structures: White-Light Emission and Beyond. J. Am. Chem. Soc. 2010, 132, 15872–15874. [Google Scholar] [CrossRef]
- Ishi-i, T.; Taguri, Y.; Kato, S.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Mataka, S. Singlet oxygen generation by two-photon excitation of porphyrin derivatives having two-photon-absorbing benzothiadiazole chromophores. J. Mater. Chem. 2007, 17, 3341. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Kudryashev, T.A.; Rakitin, O.A. 4,7-Bis(dodecylthio)-[1,2,5]thiadiazolo[3,4-c]pyridine. Molbank 2021, 2021, M1291. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Wu, W.; Chmovzh, T.N.; Robertson, N.; Woollins, J.D.; Rakitin, O.A. Dye-sensitized solar cells: Investigation of D-A-π-A organic sensitizers based on [1,2,5]selenadiazolo[3,4-c]pyridine. Sol. Energy 2017, 144, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Bolshakov, O.I.; Grishina, M.A.; Galushko, A.S.; Potemkin, V.A.; Rakitin, O.A. Design and theoretical study of D–A–π–A organic sensitizers with [1,2,5]thiadiazolo[3,4-c]pyridine, [1,2,5]selenadiazolo[3,4-c]pyridine, [1,2,5]oxadiazolo[3,4-c]pyridine π component. J. Chem. 2015, 2015, 467593. [Google Scholar] [CrossRef] [Green Version]
- Goossen, L.J.; Rodríguez, N.; Melzer, B.; Linder, C.; Deng, G.; Levy, L.M. Biaryl Synthesis via Pd-Catalyzed Decarboxylative Coupling of Aromatic Carboxylates with Aryl Halides. J. Am. Chem. Soc. 2007, 129, 4824–4833. [Google Scholar] [CrossRef]
- Welch, G.C.; Perez, L.A.; Hoven, C.V.; Zhang, Y.; Dang, X.-D.; Sharenko, A.; Toney, M.F.; Kramer, E.J.; Nguyen, T.-Q.; Bazan, G.C. A modular molecular framework for utility in small-molecule solution-processed organic photovoltaic devices. J. Mater. Chem. 2011, 21, 12700. [Google Scholar] [CrossRef]
- Henson, Z.B.; Welch, G.C.; van der Poll, T.; Bazan, G.C. Pyridalthiadiazole-Based Narrow Band Gap Chromophores. J. Am. Chem. Soc. 2012, 134, 3766–3779. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Zhang, Y.; Chen, K.-S.; Zeigler, D.F.; Chen, F.-C.; Lin, B.; Jen, A.K.-Y. High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo[3,4-c]pyridine units for thin film transistor and photovoltaic applications. J. Mater. Chem. 2011, 21, 13247–13255. [Google Scholar] [CrossRef]
- McKeown, N.B.; Badriya, S.; Helliwell, M.; Shkunov, M. The synthesis of robust, polymeric hole-transport materials from oligoarylamine substituted styrenes. J. Mater. Chem. 2007, 17, 2088–2094. [Google Scholar] [CrossRef]
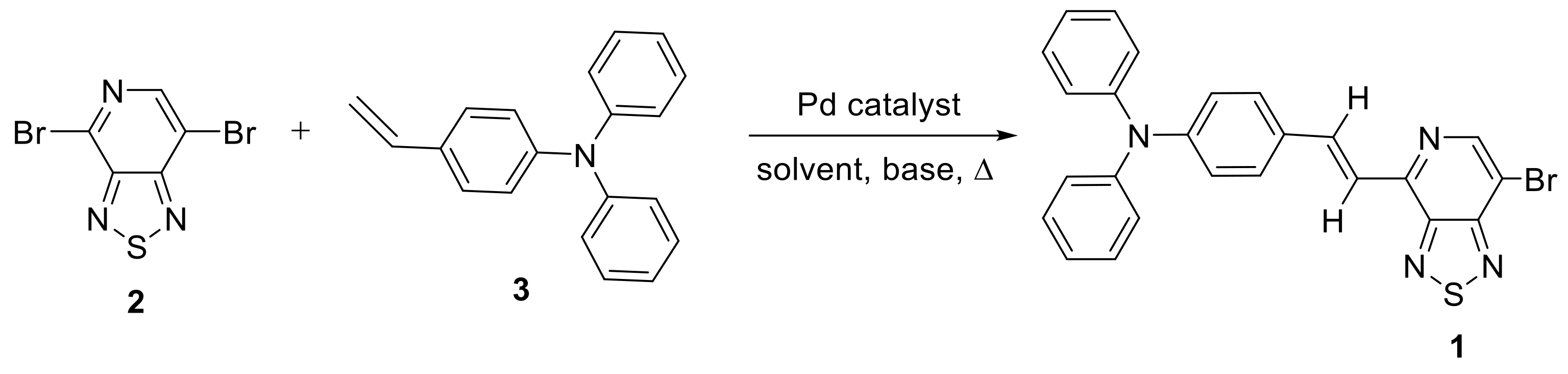
| Entry | Catalyst | Base | Solvent | Temperature, °C | Time, h | Yield, of 3% |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | K2CO3 | DMF | 80 | 8 | 0 |
| 2 | Pd(PPh3)4 | K2CO3 | DMF | 80 | 10 | 0 |
| 3 | Pd(PPh3)4 | K2CO3 | Toluene | 110 | 10 | 3 |
| 4 | Pd(PPh3)4 | Et3N | Toluene | 110 | 48 | 38 |
| 5 | Pd(PPh3)4 | Et3N | Toluene | 110 | 64 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Rakitin, O.A. (E)-4-(2-(7-Bromo-[1,2,5]thiadiazolo[3,4-c]pyridin-4-yl)vinyl)-N,N-diphenylaniline. Molbank 2022, 2022, M1368. https://doi.org/10.3390/M1368
Chmovzh TN, Rakitin OA. (E)-4-(2-(7-Bromo-[1,2,5]thiadiazolo[3,4-c]pyridin-4-yl)vinyl)-N,N-diphenylaniline. Molbank. 2022; 2022(2):M1368. https://doi.org/10.3390/M1368
Chicago/Turabian StyleChmovzh, Timofey N., and Oleg A. Rakitin. 2022. "(E)-4-(2-(7-Bromo-[1,2,5]thiadiazolo[3,4-c]pyridin-4-yl)vinyl)-N,N-diphenylaniline" Molbank 2022, no. 2: M1368. https://doi.org/10.3390/M1368
APA StyleChmovzh, T. N., & Rakitin, O. A. (2022). (E)-4-(2-(7-Bromo-[1,2,5]thiadiazolo[3,4-c]pyridin-4-yl)vinyl)-N,N-diphenylaniline. Molbank, 2022(2), M1368. https://doi.org/10.3390/M1368







