4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Rakitin, O.A. Recent developments in the synthesis of 1,2,5-thiadiazoles and 2,1,3-benzothiadiazoles. Synthesis 2019, 51, 4338–4347. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Ono, K.; Tanaka, S.; Yamashita, Y. Benzobis(thiadiazole)s Containing Hypervalent Sulfur Atoms: Novel Heterocycles with High Electron Affinity and Short Intermolecular Contacts between Heteroatoms. Angew. Chem. Int. Ed. Engl. 1994, 33, 1977–1979. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Amelichev, S.A.; Rakitin, O.A. Synthesis of 4,7-dibromo derivative of ultrahigh electron-deficient [1,2,5]thiadiazolo [3,4-d]pyridazine heterocycle and its cross-coupling reactions. Eur. J. Org. Chem. 2018, 41, 5668–5677. [Google Scholar] [CrossRef]
- Wang, Y.; Kadoya, T.; Wang, L.; Hayakawa, T.; Tokita, M.; Mori, T.; Michinobu, T. Benzobisthiadiazole-based conjugated donor–acceptor polymers for organic thin film transistors: Effects of π-conjugated bridges on ambipolar transport. J. Mater. Chem. C 2015, 3, 1196–1207. [Google Scholar] [CrossRef]
- Du, X.; Qi, J.; Zhang, Z.; Ma, D.; Wang, Z.Y. Efficient Non-Doped Near Infrared Organic Light-Emitting Devices Based on Fluorophores with Aggregation-Induced Emission Enhancement. Chem. Mater. 2012, 24, 2178–2185. [Google Scholar] [CrossRef]
- Bianchi, L.; Zhang, X.; Chen, Z.; Chen, P.; Zhou, X.; Tang, Y.; Liu, B.; Guo, X.; Facchetti, A. New Benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (iso-BBT)-Based Polymers for Application in Transistors and Solar Cells. Chem. Mater. 2019, 31, 6519–6529. [Google Scholar] [CrossRef]
- Facchetti, A.; Chen, Z.; Brown, J.E. Semiconducting Compounds and Related Devices. U.S. Patent, 0110666, 2017. [Google Scholar]
- Konstantinova, L.S.; Knyazeva, E.A.; Rakitin, O.A. Recent Developments in the Synthesis and Applications of 1,2,5-Thia- and Selenadiazoles. A Review. Org. Prep. Proced. Int. 2014, 46, 475–544. [Google Scholar] [CrossRef]
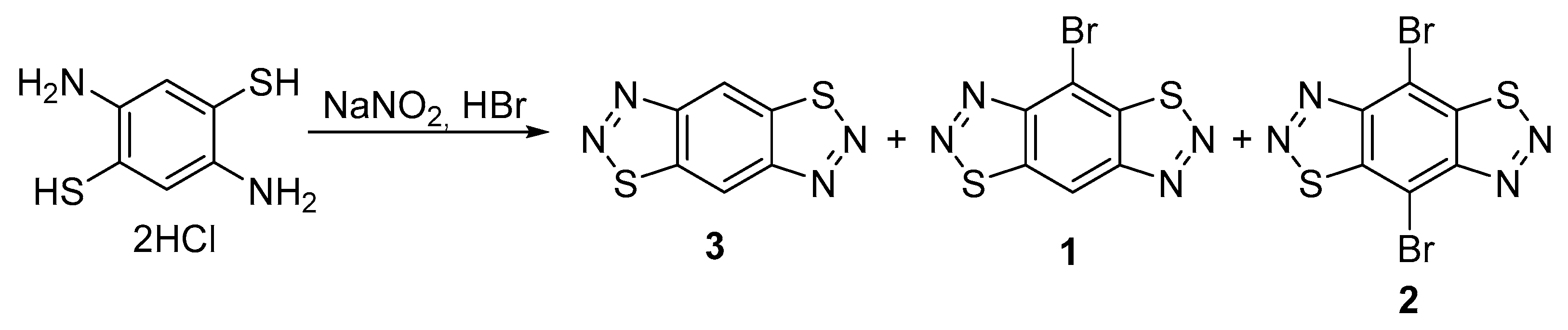
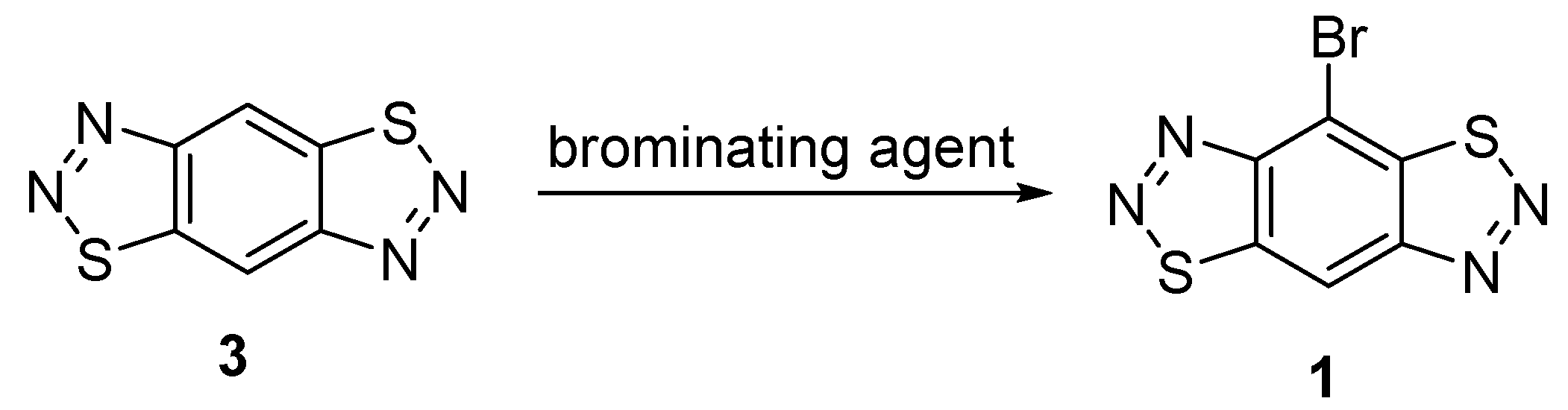
| Entry | Reagent | Solvent | Temperature, °C | Time, h | Yield of 1% |
|---|---|---|---|---|---|
| 1 | NBS | CHCl3 | 25 | 24 | 0 |
| 2 | NBS | DMF | 25 | 24 | 0 |
| 3 | NBS | CHCl3 | 61 | 24 | 0 |
| 4 | NBS | DMF | 110 | 24 | 0 |
| 5 | Br2 | HBr | 80 | 12 | 60 |
| 6 | Br2 | HBr | 110 | 12 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Alekhina, D.A.; Kudryashev, T.A.; Rakitin, O.A. 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole). Molbank 2022, 2022, M1362. https://doi.org/10.3390/M1362
Chmovzh TN, Alekhina DA, Kudryashev TA, Rakitin OA. 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole). Molbank. 2022; 2022(2):M1362. https://doi.org/10.3390/M1362
Chicago/Turabian StyleChmovzh, Timofey N., Daria A. Alekhina, Timofey A. Kudryashev, and Oleg A. Rakitin. 2022. "4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)" Molbank 2022, no. 2: M1362. https://doi.org/10.3390/M1362
APA StyleChmovzh, T. N., Alekhina, D. A., Kudryashev, T. A., & Rakitin, O. A. (2022). 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole). Molbank, 2022(2), M1362. https://doi.org/10.3390/M1362








