Abstract
8-Aminoquinoline amides of 3-oxo-olean-12-en-28-oic acid and 3-oxo-urs-12-en-28-oic acid were obtained and characterized by 1H, 13C-NMR and single crystal X-ray analysis. The used triterpenoic acids are oxidized forms of naturally occurring oleanolic acid and ursolic acids. Such types of derivatives are known for their anticancer and antiviral activities. On the other hand, 8-aminoquinoline amides are frequently used for transition metal complexation that is applicable for both C-H activation processes and biological activity studies.
1. Introduction
Many naturally occurring pentacyclic triterpenoids are known as important secondary metabolites, which exhibit significant biological activities. The most representative compounds of this family are oleanolic, ursolic and betulinic acids, which are present in many medicinal plants [1,2]. These triterpenoic acids show remarkable antitumor [3,4,5,6], antidiabetic [7,8], anti-inflammatory [9,10] and antiviral [11] properties. Oleanolic and ursolic acids’ structure contains two functional groups that can be easily modified: hydroxyl group at C(3) and carboxyl group at C(28). A possible option of further functionalization of the carboxylic moiety is amidation. In the last few decades, several dozen new ursolic and oleanolic acid amides containing alkyl, aromatic and heteroaromatic moieties have been reported [12,13,14,15,16,17,18,19]. Typically, synthesis of secondary amides of triterpenoic acids is based on the conversion of triterpenoic acid to corresponding acyl chloride in the presence of oxalylchloride in DCM and following addition of amine in the presence of base (e.g., triethylamine).
Czuk’s group discovered few triterpenoic acid 4-aminoisoquinoline and 5-aminoquinoline amide derivatives, which exhibit high cytotoxicity for human tumor cell lines, but remain significantly less cytotoxic for the mouse fibroblasts NIH 3T3 [20]. Such a high selectivity can be explained by the presence of isoquinoline and quinoline moiety, which are known biologically active heterocycles [21,22].
On the other hand, oxidation of the hydroxyl group to ketone can significantly improve several biological properties of triterpenoic acids. Thus, ursonic acid is more efficient towards a wide spectrum of biological targets than ursolic acid [23]. Nevertheless, amides of ursonic and oleanonic acid have not been widely studied. Wang’s group reported several aniline amides of ursonic acid as potential apoptosis inhibitors [24].
Hence, we decided to assemble novel ursonic and oleanonic acid 8-aminoquinoline amides. This arrangement of the quinoline ring could improve not only biological applications of triterpenoid derivatives, but also could become a directing auxiliary and open up new potential opportunities for functionalization of unreactive sites at the triterpenoid core [25]. The placement of 8-aminoquinoline amide moiety in these compounds is highly favorable also for formation of stable complexes with transition metals; thereby, it may increase the biological and synthetic application of target molecules.
2. Results and Discussion
The hydroxyl group at C(3) of triterpenoic acid can be easily converted to ketone using selective oxidants such as Jones reagent, Dess–Martin periodinane or pyridinium chlorochromate (PCC). For that purpose, PCC was chosen due to the mild reaction conditions and the most convenient purification procedure. The obtained ketoacids [26] 1a and 1b were converted into corresponding acyl chlorides 2 by a treatment with oxalyl chloride. After full conversion of the starting material (HPLC analysis), oxalyl chloride excess was removed from the reaction mixture by full evaporation. Further addition of freshly prepared triterpenoic acid chloride to a cooled solution of 8-aminoquinoline, triethylamine and DMAP led to the formation of the desired products 3a and 3b with yields of 80% and 84%, respectively (Scheme 1).
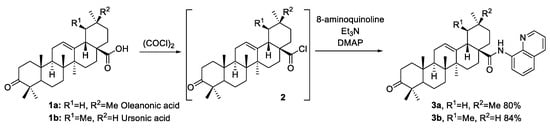
Scheme 1.
Synthesis of target compounds 3a and 3b.
The molecular structure of compound 3a was unambiguously established by single-crystal X-ray diffraction analysis (Figure 1). The X-ray analysis revealed two possible conformations of compound 3b in its solid state. They both exhibit previously known geometry of ursane and oleane aliphatic polycycles. However, the location of the heteroaromatic part differs by a torsion angle C18A-C17A-C28A-N9A′, which is −12.37(2)° for conformation I and −4.53(2) ° for conformation II. For both conformers, the planar quinoline moiety is situated almost perpendicularly (80–83°) against the least squares of the aliphatic polycyclic skeleton (C/D cycles). X-ray analysis of product 3a also showed that 8-aminoquinoline amide moiety occupies conformation, which can affect the NMR chemical shift of protons at C(11) and methyl group protons at C(8) due to anisotropic shielding by the aromatic system. Indeed, further solution NMR studies showed that H-C(11) and H3C-C(8) are shielded if compared with starting materials 1a,b that do not contain such an aromatic system. On the other hand, H-C(12) is deshielded as it points nearly perpendicularly to H-C(11). Such an average conformation in the solution is proved also by both 2D-NOESY (mixing time 300 ms) and 1D-NOE interactions that clearly indicate the trough–space interaction of the indicated aromatic system and H-C(11) and H3C-C(8) (Figure 1). This was further supported by 1D-NOEDIFF spectra as the relative values of the observed NOE effects correlate with the intensity of cross peaks in the 2D-NOESY spectrum (Figure 1A). Thus, the 1D-NOEDIFF spectrum was acquired with presaturation at 8.81 ppm for H-C(2′) (6 s, 50 dB; on-resonance) and 4.50 ppm (off-resonance reference), and the intensity difference for the H-C(11) signal at 1.97 ppm was calculated as 1.3%. Next, the presaturation at 0.54 ppm for H3C-C(8) (8 s, 40 dB; on-resonance) and 4.50 ppm (off-resonance reference) revealed intensity differences for the H-C(2′) signal at 8.81 ppm (0.6%) and the H-C(7′) signal at 8.85 ppm (1.6%). In addition to that, the 8-aminoquinoline substituent shows typical chemical shifts for both the Daugulis-directing group (heteroaromatic signals)25 and triterpenoid-derived quinoline amides (amide NH signal) [20].
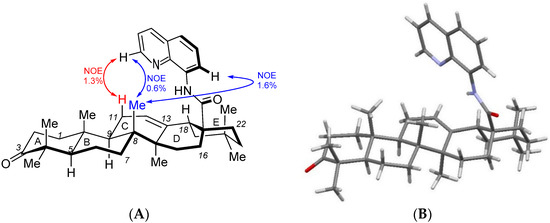
Figure 1.
Comparison of NOE effects observed in solution (A) and molecular structure obtained by single-crystal X-ray analysis (B) of compound 3a.
3. Materials and Methods
Dichloromethane for the reactions was dried over CaH2 and freshly distilled prior to use. All purchased chemicals (Angene, Fluorochem, Hadfield, UK) were used as received. All reactions were followed by TLC on E. Merck Kieselgel 60 F254 (Merck & Co., Inc., Kenilworth, NJ, USA) and visualized by using UV lamp. Column chromatography was performed on silica gel (60 Å, 40–63 μm, UPAG-AG). 1H and 13C-NMR spectra were recorded on a Bruker Avance 500 MHz (Bruker Corporation, Billerica, MA, USA), in CDCl3 at 25 °C. Chemical shift (δ) values are reported in ppm. The residual solvent peaks are used as an internal reference (CDCl3 7.26 ppm for 1H-NMR, CDCl3 77.16 ppm for 13C-NMR), s (singlet), d (doublet), t (triplet), m (multiplet); J in hertz.
To a solution of known ketocarboxylic acid26 1a or 1b (1.300 g, 2.86 mmol, 1 eq.) in anhydrous DCM (14 mL), oxalyl chloride (0.370 mL, 4.29 mmol, 1.5 eq.) was added dropwise at 0–5 °C and stirred for 10 min. The resulting mixture was allowed to warm up to room temperature and left stirring for 2 h. The solvent and the residual oxalyl chloride were removed under reduced pressure. The obtained residue was dissolved in anhydrous DCM (14 mL), and then it was added dropwise to a solution of 8-aminoquinoline (0.452 g, 3.14 mmol, 1 eq.), DMAP (0.003 g, 0.029 mmol, 0.01 eq.) and triethylamine (0.516 mL, 3.72 mmol, 1.3 eq.) in anhydrous DCM (14 mL) at 0–5 °C. The resulting reaction mixture was allowed to warm up to room temperature and stirred overnight.
The reaction mixture was quenched with 2% aqueous hydrochloric acid (50 mL). Organic layer was separated and washed with 2% aqueous hydrochloric acid (2 × 50 mL), brine (40 mL) and dried over anhydrous Na2SO4. Then, it was filtered and the solvent was removed under reduced pressure. Crude product was purified by column chromatography (eluent 20% DCM/hexanes → 100% DCM) to obtain pure triterpenoic acid 8-aminoquinoline amides 3a and 3b.
- 3-Oxo-olean-12-en-28-oic acid 8-aminoquinoline amide3a. Yield of 80% (1.321 g) as a white amorphous solid. Single crystals of amide 3a, which are suitable for X-ray analysis, were obtained by slow evaporation from DCM/hexane’s mixture with m.p. 238–239 °C. Rf = 0.42 (Hex/EtOAc 4:1). 1H-NMR (500 MHz, CDCl3) δ 10.37 (s, 1H, H-N), 8.85 (dd, 3J = 7.7 Hz, 4J = 1.7 Hz, 1H, H-C(7′)), 8.81 (dd, 3J = 4.2 Hz, 4J = 1.7 Hz, 1H,H-C(2′)), 8.15 (dd, 3J = 8.2 Hz, 4J = 1.7 Hz, 1H, H-C(4′)), 7.52 (dd, 3J = 8.3, 7.7 Hz, 1H, H-C(6′)), 7.47 (dd, 3J = 8.3 Hz, 4J = 1.7 Hz, 1H, H-C(5′)), 7.45 (dd, 3J = 8.2 Hz, 3J = 4.2 Hz, 1H, H-C(3′)), 5.73 (t, 3J = 3.7 Hz, 1H, H-C(12)), 3.01 (dd, 3J = 12.9 Hz, 4J = 3.7 Hz, 1H, H-C(18)), 2.50 (ddd, 2J = 15.9 Hz, 3J = 11.1, 7.3 Hz, 1H, Ha-C(2)), 2.34 (ddd, 2J = 15.9 Hz, 3J = 6.8, 3.7 Hz, 1H, Hb-C(2)), 2.16 (td, 2J = 13.5 Hz,3J = 3.7 Hz, 1H, Ha-C(16)), 1.97 (m, 2H, H2-C(11)), 1.92–1.79 (m, 5H, Hb-C(16), Ha-C(1), Ha-C(19), H2C(22)), 1.74 (ddd, 2J = 14.1 Hz, 3J = 13.3, 4.2 Hz, 1H, Ha-C(15)), 1.66 (dd, 3J = 9.0, 8.6 Hz, 1H, H-C(9)), 1.52–1.23 (m, 9H, H-C(5),H2C(7), Hb-C(1), H2-C(6), Hb-C(19), H2C(21)), 1.22 (s, 3H, H3-C(27)), 1.13 (ddd, 2J = 14.1 Hz, 3J = 6.7, 3.7 Hz, 1H, Hb-C(15)), 1.04 (s, 3H, H3-C(23)), 0.99 (s, 3H, H3-C(29)), 0.96 (s, 3H, H3-C(30)), 0.95 (s, 3H, H3-C(24)), 0.85 (s, 3H, H3-C(25), 0.54 (s, 3H, H3-C(26)). 13C-NMR (125.6 MHz, CDCl3) δ 217.71 (C3), 176.95(O=C-NH), 147.84(C2′) 143.25 (C13), 139.02(C8a′), 136.23 (C4′), 134.94 C(8′), 127.98 (C4a′), 127.56(C6′), 123.87(C12), 121.44 (C3′), 121.13 (C5′), 116.38(C7′), 55.25 (C5), 48.13 (C17), 47.42 (C4), 46.89 (C9), 46.77 (C19), 42.27 (C18), 41.98 (C14), 39.43 (C8), 39.19 (C1), 36.63 (C10), 34.32 (C21), 34.15 (C2), 33.11 (C30), 32.97 (C22), 31.96 (C7), 30.81 (C20), 27.58 (C15), 26.40 (C23), 25.88 (C27), 24.15 (C16), 23.70 (C29), 23.61 C(11), 21.40 (C24), 19.47 (C6), 16.23 (C26), 15.00 (C25). IR (FTIR): 3436 (s), 3333 (s), 2942 (s), 2863 (m), 1702 (s), 1673 (s), 1532 (s), 1487 (m), 1462 (m), 1424 (m), 1384 (m), 1326 (m), 1261 (w), 1164 (m), 1074 (w), 999 (w), 826 (m), 792 (m), 771 (w), 678 (w) cm−1. HRMS (ESI): m/z calcd. for [C39H52N2O2+H]+ 581.4107; found 581.4116.
- 3-Oxo-urs-12-en-28-oic acid 8-aminoquinoline amide3b. Yield of 84% (1.385 g) as a white amorphous solid. Rf = 0.40 (Hex/EtOAc 4:1). 1H-NMR (500 MHz, CDCl3) δ 10.30 (s, 1H,N-H), 8.85 (dd, 3J = 7.7 Hz, 4J = 1.5 Hz, 1H,H-C(7′)), 8.82 (dd, 3J = 4.0 Hz, 4J = 1.5 Hz, 1H, H-C(2′)), 8.15 (dd, 3J = 8.2 Hz, 4J = 1.5 Hz, 1H, H-C(4′)), 7.53 (dd,3J = 8.1, 7.7 Hz, 1H, H-C(6′)), 7.49 (dd 3J = 8.1 Hz, 4J = 1.5 Hz, 1H, H-C(5′), 7.47 (dd, 3J = 8.2, 4.0 Hz, 1H, H-C(3′)), 5.70 (t, 3J = 3.8 Hz, 1H, H-C(12)), 2.51 (ddd, 2J = 15.9 Hz 3J = 10.9, 7.3 Hz, 1H, Ha-C(2)), 2.41–2.34 (m, 2H, Hb-C(2), H-C(18)), 2.17 (td, 2J = 13.7 Hz, 3J = 4.3 Hz, 1H, Ha-C(16)), 2.09–1.81 (m, 6H, Ha-C(1), Ha-C(15), Hb-C(16), H2C-(11), Ha-C(22)), 1.70 (ddd, 2J = 13.8 Hz, 3J = 13.5, 4.1 Hz, 1H, Hb-C(22)), 1.67–1.26 (m, 10H, Hb-C(1), H-C(5), H2-C(6), H2-C(7), H2-C(21), H-C(19), H-C(9)), 1.18 (s, 3H, H3-C(27)), 1.17–1.08 (m, 2H, Hb-C(15), H-C(20)), 1.07 (s, 3H, H3-C(23)), 1.03 (s, 3H, H3-C(30)), 1.01 (s, 3H, H3-C(29)), 0.97 (s, 3H, H3-C(24)), 0.83 (s, 3H, H3-C(25)), 0.54 (s, 3H, H3-C(26)). 13C-NMR (125.6 MHz, CDCl3) δ 217.65(C3), 176.74 (O=C-NH), 147.70(C1′), 138.94 (C8a′), 137.75(C13), 136.14(C4′), 134.89 (C8′), 127.87(C4a′), 127.46 (C6′), 126.89 (C3′), 121.31 (C12), 120.96 (C5′), 116.31 (C7′), 55.09 (C5), 53.70 (C18), 49.43 (C17), 47.27 (C4), 46.68 (C9), 42.21 (C14), 39.81 (C19), 39.44 (C8), 39.19 (C1), 38.94 (C20), 37.38 (C22), 36.45 (C10), 34.04 (C2), 32.10 (C7), 30.95 (C21), 27.89 (C15), 26.39 (C23), 25.08 (C16), 23.45 (C27), 23.29 (C11), 21.29 (C24), 21.15 (C23), 19.36 (C6), 17.17 (C29), 16.19 (C26), 15.03 (C25). IR (FTIR): 3367 (s), 2927 (s), 2868 (s), 1705 (s), 1668 (s), 1526 (s), 1486 (s), 1458 (m), 1424 (m), 1383 (s), 1324 (m), 826 (m), 792 (m), 671(w), 663 (w) cm−1. HRMS (ESI): m/z calcd. for [C39H52N2O2+H]+ 581.4107; found 581.4124.
Single-crystal diffraction data for oleanolic derivative 3a were collected on an XtaLAB Synergy-S Dualflex diffractometer (Rigaku Corporation, Tokyo, Japan) equipped with a HyPix6000 detector and micro-focus-sealed X-ray tube using Cu Kα radiation (λ = 1.54184 Å). Single crystals were fixed with oil in a nylon loop of a magnetic CryoCap and set on a goniometer head. The samples were cooled down to 150 K, and ω-scans were performed with a step size of 0.5°. Data collection and reduction were performed with the CrysAlisPro 1.171.40.35a software (Oxford Diffraction Ltd., Abingdon, UK). Structure solution and refinement were performed with SHELXT and SHELXL software that are parts of the CrysAlisPro and Olex2 suites.
Full crystallographic data for compound 3a were deposited with the Cambridge Crystallographic Data Center as a supplementary publication No. CCDC-2159312 (See the Supplementary Materials). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk). Crystal data for compound 3a (C39H52N2O2; M = 580.83 g/mol): monoclinic, space group P21 (no. 4), a = 7.3438(1) Å, b = 25.6051(3) Å, c = 17.3123(3) Å, β = 100.256(1) °, V = 3203.37(8) Å3, Z = 4, T = 150.0(2) K, μ(CuKα) = 0.56 mm−1, Dcalc = 1.204 g/cm3, 30,249 reflections measured (5.2° ≤ 2Θ ≤ 153.2°), 10,724 unique (Rint = 0.042, Rsigma = 0.044) which were used in all calculations. The final R1 was 0.035 (I > 2σ(I)) and wR2 was 0.090 (all data).
4. Conclusions
Oleanonic acid (3-oxo-olean-12-en-28-oic acid 8-aminoquinoline amide 3a) and ursonic acid (3-oxo-urs-12-en-28-oic acid 8-aminoquinoline amide 3b) were successfully synthesized from the corresponding acyl chlorides and fully characterized by 1H-NMR and 13C-NMR spectroscopy. Combined-solution NOESY spectroscopy and single-crystal X-ray analysis of amide 3a revealed that the aminoquinoline moiety leans over the pentacyclic core and is located closer to the C/D cycles than to the E cycle.
Supplementary Materials
The following supporting information are available online. Figure S1: 1H-NMR spectrum of 3a; Figure S2: 13C-NMR spectrum of 3a; Figure S3: IR spectrum of 3a; Figure S4: Mass spectrum of 3a; Figure S5: 1H-NMR spectrum of 3b; Figure S6: 13C-NMR spectrum of 3b; Figure S7: IR spectrum of 3b; Figure S8: Mass spectrum of 3b. CheckCIF report and *.cif file for compound 3a as separate files.
Author Contributions
V.K. and J.L. conducted synthetic experiments and prepared the manuscript; M.T. brought the idea, managed the research and reviewed the manuscript; A.M. performed and described the X-ray studies. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Social Fund within the Project No 8.2.2.0/20/I/008 “Strengthening of PhD students and academic personnel of Riga Technical University and BA School of Business and Finance in the strategic fields of specialization” of the Specific Objective 8.2.2 “To Strengthen Academic Staff of Higher Education Institutions in Strategic Specialization Areas” of the Operational Programme “Growth and Employment”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yeung, M.F. A review on presence of oleanolic acid in natural products. Nat. Prod. Med. 2009, 2, 77–290. [Google Scholar]
- Ramsay, K.S.; Wafo, P.; Ali, Z.; Khan, A.; Oluyemisi, O.O.; Marasini, B.P.; Khan, I.A.; Bonaventure, N.T.; Choudhary, M.I.; Atta-ur-Rahman. Chemical con-stituents of Stereospermum acuminatissimum and their urease and alpha-chymotrypsin inhibitions. Fitoterapia 2012, 83, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; You, H.J.; Jeong, H.G. Nitric oxide and tumor necrosis factor-a production by oleanolic acid via nuclear factor-kB activation in macrophages. Biochem. Biophys. Res. Commun. 2001, 288, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Mieriņa, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-bark Triterpenoids and their derivatives in anticancer research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.D.; Avram, Z.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer potential of betulonic acid derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic acid enhances insulin secretion in pancreatic b-cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.I.; Lee, M.K. Ursolic acid enhances the cellular immune system and pancreatic beta-cell function in streptozotocin-induced diabetic mice fed a high-fat diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef]
- Huguet, A.I.; del Carmen Recio, M.; Máñez, S.; Giner, R.M.; Ríos, J.L. Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur. J. Pharmacol. 2000, 410, 69–81. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cwynar, B.B. Anilides and Toluidides of 3β-Acetyloleanolic Acid. Nat. Prod. Commun. 2012, 7, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Honda, Y.; Favaloro, F.G., Jr.; Gribble, G.W.; Suh, N.; Place, A.E.; Rendi, M.H.; Sporn, M.B. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg. Med. Chem. Lett. 2002, 12, 1027–1030. [Google Scholar] [CrossRef]
- Li, J.F.; Zhao, Y.; Cai, M.M.; Li, X.F.; Li, J.X. Synthesis and evaluation of a novel series of heterocyclic oleanolic acid derivatives with anti-osteoclast formation activity. Eur. J. Med. Chem. 2009, 44, 2796–2806. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Shen, J.K.; Wang, H.K.; Cosentino, L.M.; Lee, K.H. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg. Med. Chem. Lett. 2011, 11, 3115–3118. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.X.; Zhao, J.; Wang, S.Z.; Pan, Y.; Tanaka, K.; Kadota, S. Synthesis and activity of oleanolic acid derivatives, a novel class of inhibitors of osteoclast formation. Bioorg. Med. Chem. Lett. 2005, 15, 1629–1632. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.Y.; Li, J.; Hu, L.H. Oleanolic acid and its derivatives: New inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg. Med. Chem. Lett. 2008, 16, 8697–8705. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kuhfs, J.; Csuk, R. Selective killing of cancer cells with triterpenoic acid amides-The substantial role of an aromatic moiety alignment. Eur. J. Med. Chem. 2016, 122, 452–464. [Google Scholar] [CrossRef]
- Khan, A.Y.; Gopinatha, S.K. Natural isoquinoline alkaloids: Binding aspects to functional proteins, serum albumins, hemoglobin, and lysozyme. Biophys. Rev. 2015, 7, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorka, A.P.; de Dios, A.; Roepe, P.D. Quinoline Drug–Heme Interactions and Implications for Antimalarial Cytostatic versus Cytocidal Activities. J. Med. Chem. 2013, 56, 5231–5246. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Sang, Y.L. Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid. Biomolecules 2020, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Hua, S.X.; Liao, Z.X.; Huang, X.C.; Wang, H.S. Side chain-functionalized aniline-derived ursolic acid derivatives as multidrug resistance reversers that block the nuclear factor-kappa B (NF-κB) pathway and cell proliferation. Med. Chem. Comm. 2017, 8, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.G.; Shabashov, D.; Daugulis, O. Highly regioselective arylation of sp3 C-H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E.; et al. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure−Activity Relationships, and X-ray Crystallographic Studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).