Abstract
This short note describes the synthesis of compound 6,6′-di-(2″-thiophenol)-2,2′-bipyridine from its methyl phenyl sulfane precursor via deprotection of the methyl groups. The product as well as the intermediate in the synthetic route have been characterized by UV-Vis spectroscopy, 1H- and 13C-NMR spectroscopy, FT-IR spectroscopy, and HR-MS analysis. This work presents a rare example of tetradentate chelators that bears pyridyl backbones and thiophenol donors for the coordination with 3d-transition metal cations.
1. Introduction
[NiFe]-hydrogenases in nature have the ability of catalyzing the protons to hydrogen (H2) reduction reaction at high rates with a small barrier of activation energy [1,2]. The active site of [NiFe]-hydrogenases is a bimetallic Ni-Fe cluster, of which the Ni and Fe metal centers are bridged by two cysteine residue thiolates (Scheme 1) [3,4,5]. Syntheses of metal complexes that mimic the structure and function of the active site of [NiFe]-hydrogenases have long been an important field of bioinorganic chemistry [6,7], and draw even more attention these days in the context of the development of a hydrogen economy [8]. The biomimetic Ni and Fe model complexes can help us to understand the catalytic mechanism of hydrogenases, whilst also inspiring the design of transition metal-based heterogeneous hydrogen evolution catalysts.
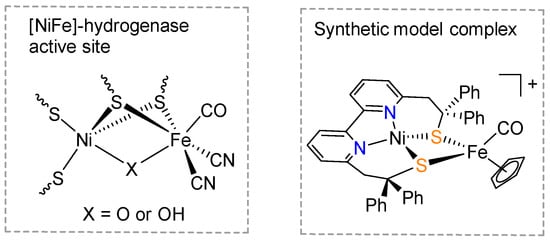
Scheme 1.
A schematic representation of the active site of [NiFe]-hydrogenase (left) and the molecular structure of a synthetic model (LN2S2NiIIFeII, right) of [NiFe]-hydrogenase [9].
Artero et al. recently reported a heterodinuclear Ni-Fe complex (Scheme 1), namely LN2S2NiIIFeII, that models the active site of [NiFe]-hydrogenases and catalyzes electrochemical H2 evolution [9,10,11]. This heterodinuclear complex was developed from the mononuclear nickel complex with the bipyridine-bisthiolate ligand, 2,2′-(2,2′-bipyridine-6,6′-diyl)bis(1,1-diphenylethanethiolate) [12,13]. Despite the successful preparation of LN2S2NiIIFeII as a unique and valuable model complex, artificial mimics for the active site of [NiFe] hydrogenase, with various structural features, are still very rare. The key challenge for reproducing the [NiFe]-hydrogenases active site in a synthetic system lies on the assembly of multiple thiolate binding sites within one organic ligand and, at the same time, in a pre-organized manner. Here, we report the synthesis of a novel organic ligand platform with bisthiophenol chelating donors, which has potential as a chelator of Ni cations in the application of syntheses of model complexes for [NiFe]-hydrogenases’ active site.
2. Results and Discussion
We designed the compound 6,6′-di-(2″-thiophenol)-2,2′-bipyridine (2 in Scheme 2) by integrating the following two design features: (i) a rigid backbone that provides coordinating sites and regulates the coordination configuration at certain extent; (ii) the availability of multiple S− donors that mimic the coordination environment around the active site of [NiFe]-hydrogenase (Scheme 1). To the best of our knowledge, 6,6′-di-(2″-thiophenol)-2,2′-bipyridine (2) is the first example of tetradentate ligands that contain both bispyridine and bisthiophenol chelating moieties. A literature survey returned one hit of compound 2 in a patent without synthetic details [14] An analogue of 1 with phenanthroline backbone has been reported before [15].
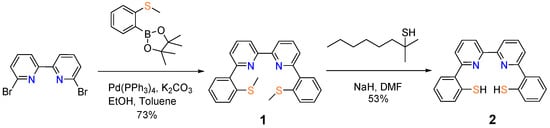
Scheme 2.
Synthesis of 6,6′-di-(2″-thiophenol)-2,2′-bipyridine.
The title compound (2) was synthesized in a two-step procedure (Scheme 2) from the commercially available starting material, 6,6′-dibromo-2,2′-bipyridine. Compound 6,6′-di-(2″-methylthiophenyl)-2,2′-bipyridine (1) was prepared under typical Suzuki–Miyaura coupling conditions using Pd(PPh3)4 as the catalyst and potassium carbonate as a base. The reaction went well in anaerobic toluene and afforded compound 1 in a yield of 73%. Deprotection of the methyl groups was first performed with NaH and tert-nonyl mercaptan in DMF at 160 °C [16]. The conventional heating condition, however, did not effectively remove the thioether substituent. Given a relatively long reaction period, TLC analysis of the reaction product revealed a collection of compounds without distinctive indication for the formation of 2. The application of a microwave reactor, which allows elevation of the reaction temperature to 200 °C, achieved the target compound 2 in a reasonable yield (53%).
Compounds 1 and 2 were both characterized by 1H- and 13C-NMR spectroscopy. The proton NMR spectrum of 1 in CDCl3 shows the signal of methyl groups as a singlet at 2.44 ppm with the integration of 6H (Figure S1). This characteristic methyl proton peak disappears in the 1H-NMR spectrum of 2. Instead, a singlet with the integration of 2H emerges at 4.57 ppm (Figure S3) and is assigned as the thiophenol protons. Elemental analysis was conducted to verify the purity of compounds 1 and 2. A high-resolution mass spectrometer was also employed to confirm the molecular formula of 1 (Figure S5). Comparing the FT-IR spectra of 1 and 2 (Figure S6) reveals the S−H stretching bands at 2506 and 2530 cm−1, which are close to the S−H stretching band of thiophenol (2545 cm−1) [17].
The UV-Vis spectra of 1 and 2 were recorded in methanol, as displayed in Figure 1. Both compounds show strong absorbance bands at λmax = 231 and 303 nm, which derive from the π → π* electron excitation at the pyridyl and phenyl moieties. The addition of one equivalent nickel acetate in the methanol solution of 2 results in significant change of the UV-Vis absorption profile: the emergence of absorbance bands at λmax = 263 and 291 nm. The phenomena suggest coordination of Ni(II) ion by the tetradentate compound 2. In contrast, the UV-Vis spectrum of 1 is not affected by the presence of nickel ion, indicating weak or no interaction between the compound Ni(II) in solution. Synthesis and isolation of 3d-transition metal complexes, particularly Ni and Fe complexes, with compound 2 as a ligand are being carried out.
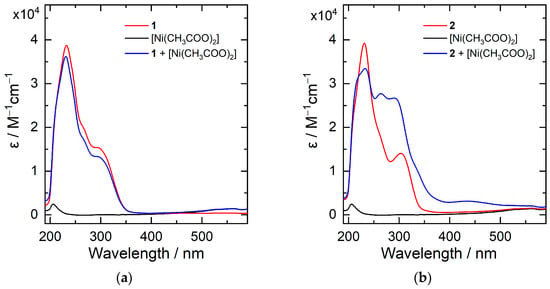
Figure 1.
UV-Vis spectra of 1 (a) and 2 (b) measured in methanol in the absence and presence of one equivalent of Ni(II) acetate.
3. Materials and Methods
All air- and moisture-sensitive experiments were performed under a dry argon atmosphere using standard Schlenk techniques. Dry solvents for moisture-sensitive experiments were purchased from commercial sources (water content ≤ 10 ppm) and used as received without further purification. 6,6′-dibromo-2,2′-bipyridine, 4,4,5,5-tetramethyl-2-(2-(methylthio)phenyl)-1,3,2-dioxaborolane, 2-methyloctane-2-thiol, and other chemicals for syntheses were commercially available and used as received. Microwave syntheses were carried out using an Anton-Parr Monowave 200 microwave reactor (Anton-Parr, Graz, Austria). Water for syntheses and analysis was purified by Milli-Q technique (18.2 MΩ, Merck, Darmstadt, Germany). Thin Layer Chromatography analyses were performed on silica gel coated glass plates with fluorescence indicator UV254. Flash column chromatography was conducted with silica gel at atmospheric pressure.
1H- and 13C-NMR spectra were recorded on a Bruker (Fällanden, Switzerland) Avance NEO (600 MHz) spectrometer, operating at a probe temperature of room temperature. Chemical shifts, δ, are reported in ppm relative to the peak of SiMe4, using 1H chemical shifts of the residual solvents as references [18]. Electronic absorption spectra were recorded with a compact OTO Photonics (Hsinchu, Taiwan) UV-Vis spectrometer (SE2030-050-FUV). High-resolution MS data were obtained using an Agilent (Santa Clara, CA, USA) 1260-6460 Q-TOF mass spectrometer. FT-IR spectra were acquired using the TENSOR II + Hyperion 2000 spectroscopy (Bruker, Ettlingen, Germany). Elemental analysis (C N H S) was performed on Vario EL Cube (Elementar, Langenselbold, Germany).
- Synthesis of 6,6′-di-(2″-methylthiophenyl)-2,2′-bipyridine (1).
4,4,5,5-Tetramethyl-2-(2-(methylthio)phenyl)-1,3,2-dioxaborolane (1.0 g, 4.0 mmol) was added to a solution of 6,6′-dibromo-2,2′-bipyridine (313 mg, 1.0 mmol) in a mixture of toluene (7 mL) and EtOH (7 mL). After degassing by Ar, K2CO3 (4.14 g, 30 mmol) and Pd(PPh3)4 (58 mg, 0.05 mmol) were added to this solution and the mixture was heated by a microwave reactor to 170 °C for 65 min under stirring. The solution was allowed to cool to room temperature and the volatile components were removed under vacuum. The residue was extracted with methylene chloride (50 mL × 3) three times. The combined organic layers were washed by saturated sodium chloride solution and then dried by anhydrous sodium sulfate. The solid salt was removed by filtration. Removal of the solvent under vacuum afforded compound 1 as an orange powder (292 mg. 73%). 1H-NMR (600 MHz, Chloroform-d) δ 8.61 (dd, J = 7.9, 1.0 Hz, 2H), 7.90 (t, J = 7.8 Hz, 2H), 7.58 (ddd, J = 10.6, 7.5, 1.2 Hz, 4H), 7.43–7.38 (m, 4H), 7.29–7.26 (m, 2H), 2.44 (s, 6H). 13C-NMR (151 MHz, Chloroform-d) δ 157.59, 155.43, 138.23, 137.45, 130.10, 129.02, 126.29, 124.88, 123.77, 120.11, 77.16, 16.92. ESI-HRMS: m/e calcd for C24H21N2S2 (M + H)+ 401.1146, found 401.1146. Mp: 215–218 °C. Anal. Calcd. for 1 (C24H20N2S2): C, 71.97; H, 5.03; N, 6.99; S, 16.01. Found: C, 71.65; H, 5.05; N, 6.73; S, 15.77.
- Synthesis of 6,6′-di-(2″-thiophenol)-2,2′-bipyridine (2).
2-Methyloctane-2-thiol (640 mg, 4.0 mmol) was added to a solution of NaH (96 mg, 4.0 mmol) in anhydrous DMF (13 mL). Compound 1 (200 mg, 0.5 mmol) was added to this solution and the mixture was stirred under an Ar atmosphere for about 10 min, until the gas bubbling ceased. The mixture was then transferred to a glass tube (designed for microwave reaction) and heated by a microwave reactor to 200 °C for 75 min under stirring. The solution was allowed to cool to room temperature, and then diluted hydrochloric acid (25 mL) was slowly dropped into it. The orange precipitate was collected by filtration and purified by column chromatography over silica using CH2Cl2 as an eluent. The pure product was obtained as an orange powder (98 mg, 53%). 1H-NMR (600 MHz, Chloroform-d) δ 8.64 (dd, J = 7.8, 1.0 Hz, 2H), 7.95 (t, J = 7.8 Hz, 2H), 7.64–7.58 (m, 4H), 7.48–7.43 (m, 2H), 7.29–7.26 (m, 4H), 4.57 (s, 2H). 13C-NMR (151 MHz, Chloroform-d) δ 138.18, 137.92, 132.21, 131.37, 130.41, 129.01, 125.73, 123.55, 120.03, 77.16. Mp: 179–182 °C. Anal. Calcd. for 2· (C22H16N2S2): C, 70.94; H, 4.33; N, 7.52; S, 17.21. Anal. Calcd. for 2·0.6H2O (C22H17.2N2O0.6S2): C, 68.94; H, 4.52; N, 7.31; S, 16.73. Found: C, 68.64; H, 4.35; N, 6.87; S, 16.87.
4. Conclusions
Compounds di-(2″-methylthiophenyl)-2,2′-bipyridine (1) and 6,6′-di-(2″-thiophenol)-2,2′-bipyridine (2) have been prepared and characterized. The deprotection of methyl groups of 1 with tert-nonyl mercaptan was achieved in DMF using a microwave reactor at 200 °C. The thiophenol bipyridine compound 2 might be used as a chelator for the Ni cation.
Supplementary Materials
The following supporting information can be downloaded: NMR spectra and HRMS analysis. Figure S1. 1H-NMR spectrum of compound 1 in CDCl3. Figure S2. 13C{1H} NMR spectrum of compound 1 in CDCl3. Figure S3. 1H-NMR spectrum of compound 2 in CDCl3. Figure S4. 13C{1H} NMR spectrum of compound 2 in CDCl3. Figure S5. HRMS spectrum of compound 1. Figure S6. FT-IR spectra of compounds 1 (blue) and 2 (red) as KBr pellets.
Author Contributions
Y.H. and L.T. conceived and designed the synthetic route, analyzed the data, and drafted the manuscript. Y.H. conducted the experiments of syntheses and characterization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangzhou University under the funding number RQ2020042.
Data Availability Statement
The data are reported in the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, A.K.; Sillery, E.; Albracht, S.P.; Armstrong, F.A. Direct comparison of the electrocatalytic oxidation of hydrogen by an enzyme and a platinum catalyst. Chem. Commun. 2002, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Frey, M. Hydrogenases: Hydrogen-Activating Enzymes. ChemBioChem 2002, 3, 153–160. [Google Scholar] [CrossRef]
- Volbeda, A.; Charon, M.-H.; Piras, C.; Hatchikian, E.C.; Frey, M.; Fontecilla-Camps, J.C. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 1995, 373, 580. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Scheerer, P.; Frielingsdorf, S.; Kroschinsky, S.; Friedrich, B.; Lenz, O.; Spahn, C.M.T. The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre. Nature 2011, 479, 249. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Nishikawa, K.; Lubitz, W. Hydrogens detected by subatomic resolution protein crystallography in a [NiFe] hydrogenase. Nature 2015, 520, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.R.; Berggren, G.; Bacchi, M.; Fontecave, M.; Artero, V. Mimicking hydrogenases: From biomimetics to artificial enzymes. Coord. Chem. Rev. 2014, 270–271, 127–150. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Dey, A. Recent developments in bioinspired modelling of [NiFe]- and [FeFe]-hydrogenases. Curr. Opin. Electrochem. 2019, 15, 155–164. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Brazzolotto, D.; Gennari, M.; Queyriaux, N.; Simmons, T.R.; Pecaut, J.; Demeshko, S.; Meyer, F.; Orio, M.; Artero, V.; Duboc, C. Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase. Nat. Chem. 2016, 8, 1054–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.E.; Chattopadhyay, S.; Wang, L.; Brazzolotto, D.; Pramanik, D.; Aldakov, D.; Fize, J.; Morozan, A.; Gennari, M.; Duboc, C.; et al. Hydrogen Evolution from Aqueous Solutions Mediated by a Heterogenized [NiFe]-Hydrogenase Model: Low pH Enables Catalysis through an Enzyme-Relevant Mechanism. Angew. Chem. Int. Ed. 2018, 57, 16001–16004. [Google Scholar] [CrossRef] [PubMed]
- Brazzolotto, D.; Wang, L.; Tang, H.; Gennari, M.; Queyriaux, N.; Philouze, C.; Demeshko, S.; Meyer, F.; Orio, M.; Artero, V.; et al. Tuning Reactivity of Bioinspired [NiFe]-Hydrogenase Models by Ligand Design and Modeling the CO Inhibition Process. ACS Catal. 2018, 8, 10658–10667. [Google Scholar] [CrossRef]
- Gennari, M.; Orio, M.; Pecaut, J.; Neese, F.; Collomb, M.N.; Duboc, C. Reversible apical coordination of imidazole between the Ni(III) and Ni(II) oxidation states of a dithiolate complex: A process related to the Ni superoxide dismutase. Inorg. Chem. 2010, 49, 6399–6401. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, C.; Hurkes, N.; Kaiser, A.; Klein, A.; Schuren, A. Electrochemistry and spectroscopy of organometallic terpyridine nickel complexes. Inorg. Chem. 2009, 48, 9947–9951. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K. White Organic Electroluminescence Device. JP Patent JP2010135689A, 17 June 2010. [Google Scholar]
- Neuhaus, J.D.; Morrow, S.M.; Brunavs, M.; Willis, M.C. Diversely Substituted Quinolines via Rhodium-Catalyzed Alkyne Hydroacylation. Org. Lett. 2016, 18, 1562–1565. [Google Scholar] [CrossRef] [Green Version]
- Manes, T.A.; Rose, M.J. Mono- and Dinuclear Manganese Carbonyls Supported by 1,8-Disubstituted (L = Py, SMe, SH) Anthracene Ligand Scaffolds. Inorg. Chem. 2016, 55, 5127–5138. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, J.H.; Yoon, S. Photooxidative coupling of thiophenol derivatives to disulfides. J. Phys. Chem. A 2010, 114, 12010–12015. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).