Abstract
Reaction of 4,5-dichloro-1,2,3-dithiazolium chloride with 2-[amino(methylthio)methylene])malononitrile (1 equiv) in the presence of pyridine (2 equiv) gave (Z)-2-{[(4-chloro-5H-1,2,3-dithiazol-5-ylidene)amino](methylthio)methylene}malononitrile in 20% yield. The compound was fully characterized.
1. Introduction
Isothiazoles are isomers of thiazoles that find uses in the medicinal (e.g., antirhinoviral, enteroviral [1,2,3,4] and anticancer activity [5], and as cathepsin C inhibitors [6]) and agrochemical (e.g., as fungicides, insecticides and acaricides [7,8,9]) sciences. Isothiazoles also have industrial applications, e.g., as dyes [10] and corrosion inhibitors [11], while they are also useful synthetic intermediates (e.g., Woodward’s synthesis of colchicine [12]). The synthesis, chemistry and applications of isothiazoles have been extensively reviewed [13,14,15].
A group of important isothiazole scaffolds are isothiazole-carbonitriles [16,17,18,19,20,21,22,23,24]. For example, isothiazole-carbonitriles 1–3 (Scheme 1) are precursors for the preparation of insecticides [25], herbicides [9] and other potent biocides [26]. Recently, we developed the synthesis of isothiazole-carbonitriles 4 starting from (4-chloro-5H-1,2,3-dithiazolylidene)acetonitriles 5, using gaseous HCl or HBr (Scheme 1) [27].
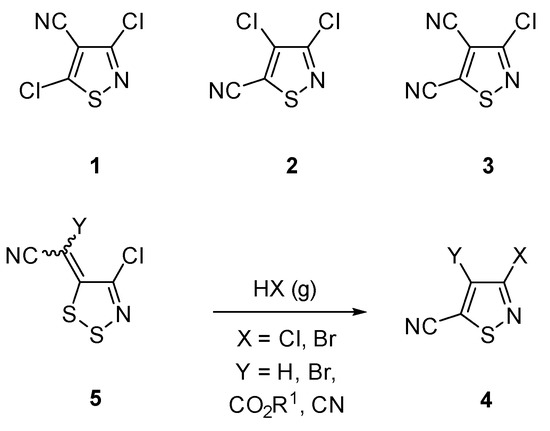
Scheme 1.
Structure of isothiazole-carbonitriles 1–3 and synthesis of isothiazoles 4 from (4-chloro-5H-1,2,3-dithiazolylidene)acetonitriles 5.
2. Results and Discussion
Another interesting synthesis of isothiazoles 6 directly from Appel’s salt 7 and primary enamines was reported by Rees [28]. In this work, two examples were reported with medium to good yields, while the reaction was proposed to proceed via the dithiazole ylidenes 8 (Scheme 2). Inspired by this work, we decided to investigate the same reaction with the analogous 2-[amino(methylthio)methylene]malononitrile that after nucleophilic addition to Appel’s salt 7 and elimination of cyanogen chloride could give ylidene 9.
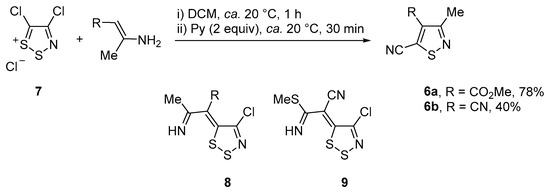
Scheme 2.
Synthesis of isothiazoles 6 from Appel’s salt 7 and structures of intermediate dithiazole ylidenes 8 and 9.
Nevertheless, the reaction of Appel’s salt 7 with 2-[amino(methylthio)methylene]malononitrile (1 equiv) in MeCN, for 1 h, followed by addition of pyridine (2 equiv) and further stirring for 24 h gave the yellow colored product (Z)-2-{[(4-chloro-5H-1,2,3-dithiazol-5-ylidene)amino](methylthio)methylene}malononitrile (10) in 20% yield together with traces of the usual Appel’s salt degradation products (Scheme 3). The desired isothiazole 6c was not observed, indicating that either the enaminic reagent preferentially reacts with Appel’s salt 7 via the nucleophilic nitrogen instead of the enamine carbon, and/or that isothiazole 6c did form but was reactive and could not be isolated.
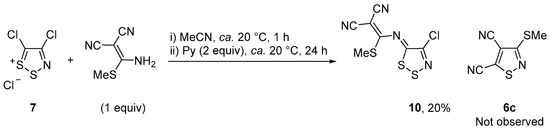
Scheme 3.
Reaction of Appel’s salt 7 with 2-[amino(methylthio)methylene]malononitrile.
Product 10 was isolated as yellow needles, mp 172–174 °C (from DCE/c-hexane). UV-vis spectroscopy supported an intact dithiazole ring (λmax 374 nm, log ε 2.99). FTIR spectroscopy showed two cyano ν(C≡N) stretches at 2224 and 2216 cm−1, while mass spectrometry revealed a molecular ion (MH +) peak of m/z 275 (100%) along with a MH++2 isotope peak at 277 (73%) that supported the presence of a single chlorine. 13C NMR spectroscopy showed the presence of one CH3 resonance and six quaternary carbon resonances (see Supplementary Materials for the complete spectra), while a correct elemental analysis (CHN) was obtained for the molecular formula C7H3ClN4S3. Tentatively, the imine geometry was assigned as Z owing to steric and electronic repulsion between the C-4 chloride and the sulfide group. Despite the low yield of its preparation, imine 10 is a multifunctional dithiazole and its chemistry will be investigated in the future. An example of the potential reactivity of this compound is the ring opening of the dithiazole by various thiophiles followed by ring closure on the cyano groups that could yield pyrimidines. Alternatively, Michael addition onto the acrylonitrile unit could then lead to cyclization on to the dithiazole C-4 position.
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler - Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a Perkin-Elmer Lambda-25 UV-vis spectrophotometer (Perkin-Elmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA) and strong, medium and weak peaks are represented by s, m and w, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 instrument [at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA)]. Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies were used for the assignment of the 13C peaks as CH3, CH2, CH and Cq (quaternary). The Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF) mass spectrum (+ve mode) was recorded on a Bruker Autoflex III Smartbeam instrument (Bruker). The elemental analysis was run by the London Metropolitan University Elemental Analysis Service. 4,5-Dichloro-1,2,3-dithiazolium chloride (7) [29] and 2-[amino(methylthio)methylene]malononitrile [30] were prepared according to the literature procedures.
(Z)-2-{[(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino](methylthio)methylene}malononitrile (10)
To a stirred suspension of 4,5-dichloro-1,2,3-dithiazolium chloride (7) (104.3 mg, 0.50 mmol) in DCM (2 mL) was added 2-[amino(methylthio)methylene]malononitrile (69.6 mg, 0.50 mmol) and the reaction mixture was stirred at ca. 20 °C for 1 h. Pyridine (81 μL, 1.00 mmol) was then added and the reaction mixture was stirred for another 24 h. The mixture was then adsorbed onto silica and chromatographed (n-hexane/DCM 20:80) to give the title compound 10 (27 mg, 20%) as yellow needles, mp 172–174 °C (from DCE/c-hexane); Rf 0.30 (n-hexane/DCM 20:80); (found: C, 30.73; H, 1.11; N, 20.33. C7H3ClN4S3 requires C, 30.60; H, 1.10; N, 20.39%); λmax(DCM)/nm 295 (log ε 3.12), 374 (2.99), 389 inf (2.93); vmax/cm−1 2933w (alkyl C–H), 2224m and 2216m (C≡N), 1572m, 1560m, 1557s, 1553s, 1528w, 1489m, 1470s, 1454w, 1416w, 1323w, 1252m, 1186m, 1123m, 988w, 935m, 924m, 901w, 870s, 839w, 810m, 716s; δH(500 MHz; CDCl3) 2.44 (3H, s, CH3); δC(125 MHz; CDCl3) 181.4 (Cq), 163.6 (Cq), 147.2 (Cq), 112.2 (Cq), 111.5 (Cq), 66.9 (Cq), 15.0 (CH3); m/z (MALDI-TOF) 277 (MH++2, 73%), 275 (MH+, 100), 239 (M+-Cl, 3), 229 (M+-SMe+2, 25), 227 (M+-SMe, 60), 204 (MH+-SMe-CN+2, 14), 202 (MH+-SMe-CN, 37), 74 (26).
Supplementary Materials
The following supporting information can be downloaded online, mol file, 1H and 13C NMR spectra.
Author Contributions
A.S.K. and P.A.K. conceived the experiments; A.S.K. performed the experiments; A.S.K. wrote the paper; A.S.K. and P.A.K. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cyprus Research Promotion Foundation, grant numbers ΣΤΡAΤHΙΙ/0308/06, NEKYP/0308/02 ΥΓΕΙA/0506/19 and ΕΝΙΣΧ/0308/83.
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: The State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd., Medisell Ltd. and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cutrì, C.C.C.; Garozzo, A.; Siracusa, M.A.; Sarvà, M.C.; Tempera, G.; Geremia, E.; Pinizzotto, M.R.; Guerrera, F. Synthesis and antiviral activity of a new series of 4-isothiazolecarbonitriles. Bioorg. Med. Chem. 1998, 6, 2271–2280. [Google Scholar] [CrossRef]
- Cutrì, C.C.C.; Garozzo, A.; Siracusa, M.A.; Sarvà, M.C.; Castro, A.; Geremia, E.; Pinizzotto, M.R.; Guerrera, F. Synthesis of New 3,4,5-Trisubstituted Isothiazoles as Effective Inhibitory Agents of Enteroviruses. Bioorg. Med. Chem. 1999, 7, 225–230. [Google Scholar] [CrossRef]
- Garozzo, A.; Cutrì, C.C.C.; Castro, A.; Tempera, G.; Guerrera, F.; Sarvà, M.C.; Geremia, E. Anti-rhinovirus activity of 3-methylthio-5-aryl-4-isothiazolecarbonitrile derivatives. Antivir. Res. 2000, 45, 199–210. [Google Scholar] [CrossRef]
- Cutrì, C.C.C.; Garozzo, A.; Siracusa, M.A.; Castro, A.; Tempera, G.; Sarvà, M.C.; Guerrera, F. Synthesis of new 3-methylthio-5-aryl-4-isothiazolecarbonitriles with broad antiviral spectrum. Antivir. Res. 2002, 55, 357–368. [Google Scholar] [CrossRef]
- Larson, E.R.; Noe, M.C.; Gant, T.G. Isothiazole Derivatives Useful as Anticancer Agents. U.S. Patent 6,235,764, 22 May 2001. [Google Scholar]
- Bullion, A.M.; Busch-Petersen, J.; Evans, B.; Neipp, C.E.; McCleland, B.W.; Nevins, N.; Wall, M.D. Cathepsin C Inhibitors. WO Patent 2011,025,799, 3 March 2011. [Google Scholar]
- Muelthau, F.A.; Bretschneider, T.; Fischer, R.; Fueslein, M.; Heil, M.; Hense, A.; Kluth, J.; Koehler, A.; Franken, E.-M.; Malsam, O.; et al. Novel Heterocyclic Compounds as Pesticides. U.S. Patent 62012,094,837, 19 April 2012. [Google Scholar]
- Sagasser, I.; Menke, O.; Rack, M.; Hamprecht, G.; Puhl, M.; Reinhard, R.; Witschel, M.; Zagar, C.; Walter, H.; Westphalen, K.-O. 3-Arylisothiazoles and Their Use as Herbicides. U.S. Patent 623,807, 15 May 2004. [Google Scholar]
- Hitoshi, S.; Yanase, Y.; Sekino, T.; Ishikawa, K.; Kuwatsuka, T.; Tanikawa, H.; Kawashima, H.; Tomura, N.; Kanemoto, Y. Isothiazolecarboxylic Acid Derivatives, Rice Blast Control Agents Containing the Same as Active Ingredients, and Rice Blast Control Method Applying the Control Agents. U.S. Patent 65,240,951, 31 August 1993. [Google Scholar]
- Bradbury, R.; Butters, A.; Moscrop, C.; Slark, A. Ink Composition. WO Patent 9,634,916, 7 November 1996. [Google Scholar]
- Pasch, N.F. Use of Corrosion Inhibiting Compounds to Inhibit Corrosion of Metal Plugs in Chemical-Mechanical Polishing. U.S. Patent 6,068,879, 30 May 2000. [Google Scholar]
- Woodward, R.B. A Total Synthesis of Colchicine; The Harvey Lecture Series; Academic Press: New York, NY, USA, 1963; Volume 59, pp. 31–47. [Google Scholar]
- Chapman, R.F.; Peart, B.J. Comprehensive Heterocyclic Chemistry II; Shinkai, I., Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; ch. 3.05; pp. 319–372. [Google Scholar]
- Potkina, V.I.; Kletskov, A.V.; Zubkov, F.I. Comprehensive Heterocyclic Chemistry IV; Black, D.S.C., Cossy, J., Stevens, C.V., Eds.; Pergamon: Oxford, UK, 2022; ch. 4.05; pp. 482–529. [Google Scholar]
- Silva, A.D.A.; McQuade, J.; Szostak, M. Recent Advances in the Synthesis and Reactivity of Isothiazoles. Adv. Synth. Cat. 2019, 361, 3050–3067. [Google Scholar] [CrossRef]
- Emayan, K.; English, R.F.; Koutentis, P.A.; Rees, C.W. New routes to benzothiophenes, isothiazoles and 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1997, 3345–3350. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Rees, C.W. Reactions of 1,2,3-dithiazoles with halogenated malononitriles. J. Chem. Soc. Perkin Trans. 1 2002, 1236–1241. [Google Scholar] [CrossRef]
- Potkin, V.I.; Zubenko, Y.S.; Nechai, N.I.; Bykhovets, A.I.; Kurman, P.V. Synthesis of 4,5-dichloro-3-cyanoisothiazole and its functional derivatives. Russ. J. Org. Chem. 2008, 44, 1038–1042. [Google Scholar] [CrossRef]
- Hatchard, W.R. The Synthesis of Isothiazoles. I. 3,5-Dichloro-4-isothiazolecarbonitrile and Its Derivatives. J. Org. Chem. 1964, 29, 660–665. [Google Scholar] [CrossRef]
- Hatchard, W.R. The Synthesis of Isothiazoles. II. 3,5-Dimercapto-4-isothiazolecarbonitrile and Its Derivatives. J. Org. Chem. 1964, 29, 665–668. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. Regioselective hydrodehalogenation of 3,5-dihaloisothiazole-4-carbonitriles: Synthesis of 3-haloisothiazole-4-carbonitriles. Tetrahedron 2011, 67, 3348–3354. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A. New regiospecific isothiazole C–C coupling chemistry. Org. Biomol. Chem. 2006, 4, 3681–3683. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kumagai, H.; Ogawa, M. Process for Producing Isothiazole Derivative. U.S. Patent 46,473, 23 February 2012. [Google Scholar]
- Zborovskii, Y.L.; Smirnov-Zamkov, I.V.; Staninets, V.I. Heterocyclization of Acetylene Derivatives by the Action of Sulfur-Dioxide and Hydrogen Bromide. Zh. Org. Khim. 1984, 20, 1774–1784. [Google Scholar]
- Turnbull, M.D.; Smith, A.M.; Salmon, R. Oxazoles and Their Agricultural Compositions. U.S. Patent 5,705,516, 6 January 1998. [Google Scholar]
- Assmann, L.; Kitagawa, Y.; Ishikawa, K.; Yamazaki, D.; Sawada, H.; Araki, Y.; Sakuma, H.; Kinbara, T.; Imanishi, K. Isothiazole carboxylic acid amides. WO Patent 15,622, 23 March 2000. [Google Scholar]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.J.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2014, 4, 7735–7748. [Google Scholar] [CrossRef]
- Clarke, D.; Emayan, K.; Rees, C.W. New synthesis of isothiazoles from primary enamines. J. Chem. Soc. Perkin Trans. 1 1998, 77–82. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und reaktionen des 4,5-dichlor-1,2,3-dithiazoliumchlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Gibbons, L.K. 3-Aminoisothiazole Derivatives as Herbicides. U.S. Patent 4,075,001, 21 February 1978. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).