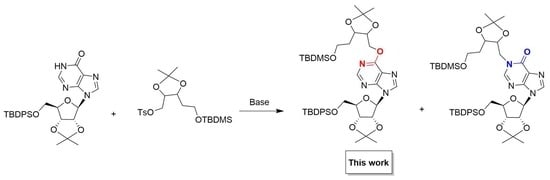
O6-[(2″,3″-O-Isopropylidene-5″-O-tbutyldimethylsilyl)pentyl]-5′-O-tbutyldiphenylsilyl-2′,3′-O-isopropylideneinosine
Abstract
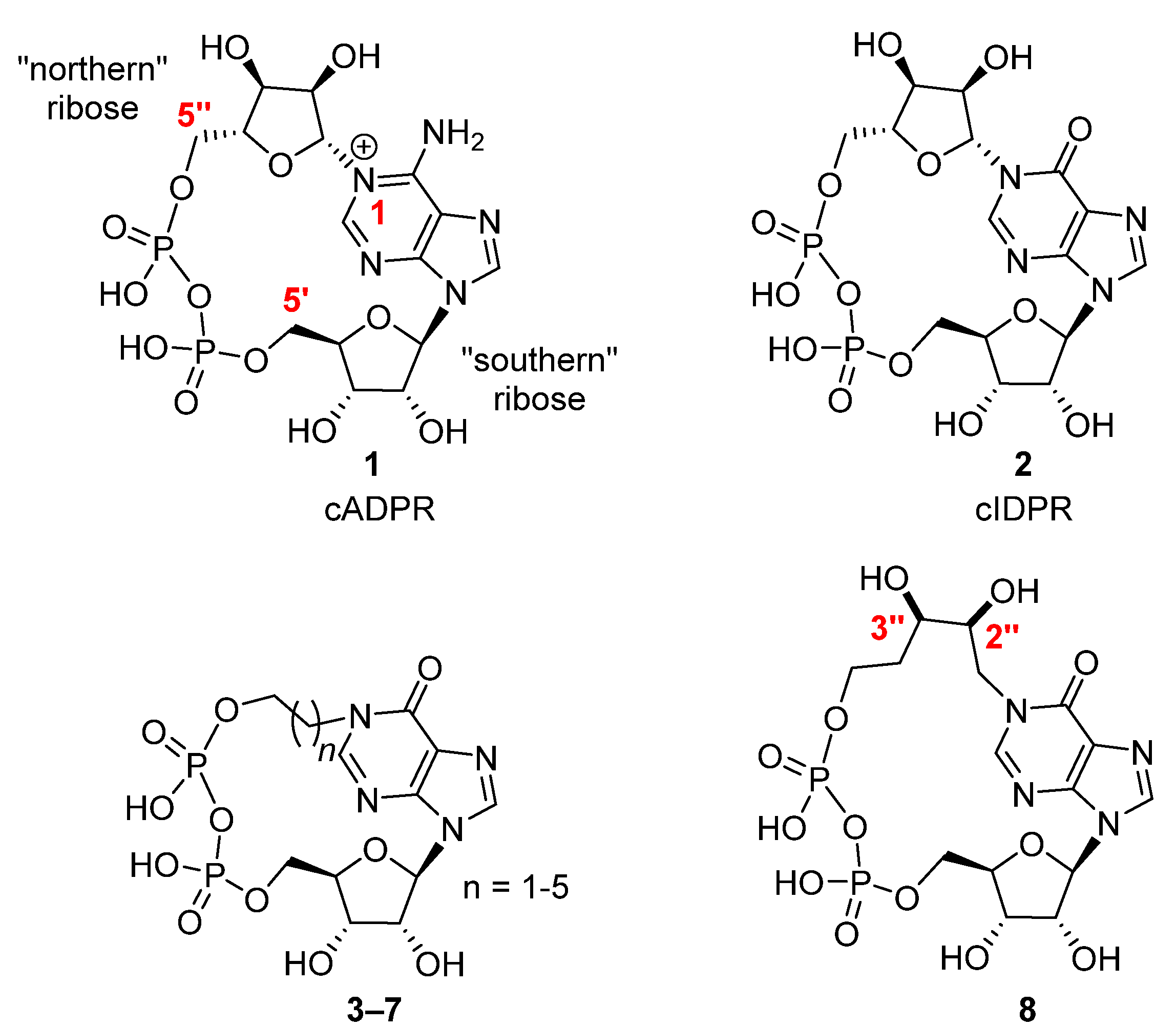
1. Introduction
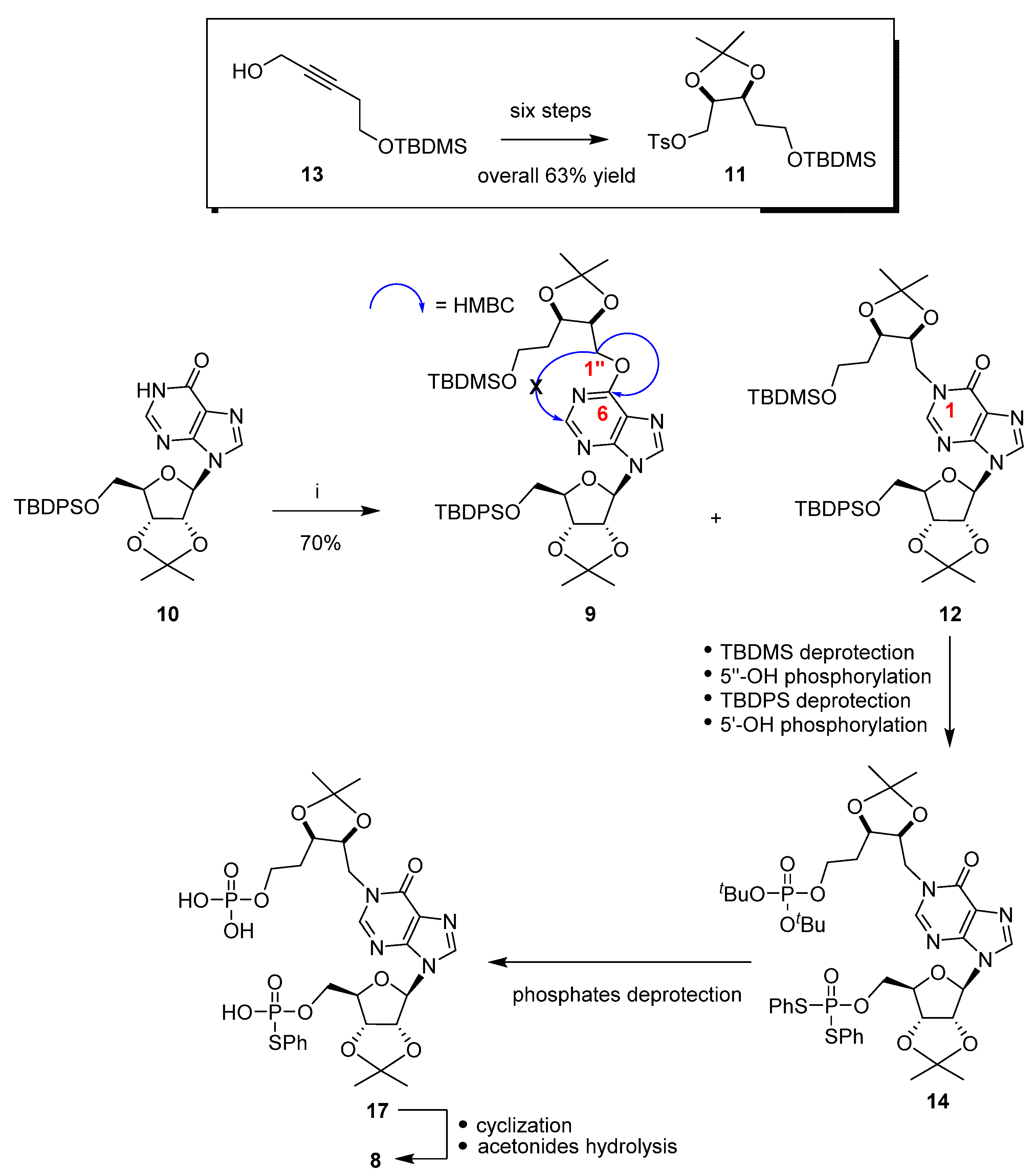
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borbone, N.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Nucleoside analogues and nucleoside precursors as drugs in the fight against SARS-CoV-2 and other coronaviruses. Molecules 2021, 26, 986. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef]
- Guinan, M.; Benckendor, C.; Smith, M.; Miller, G.J.; Way, S.; Derek, J.; Mcphee, J. Evaluation Analogues Evaluation of Anticancer Anticancer Nucleoside. Molecules 2020, 25, 2050. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ewald, B.; Sampath, D.; Plunkett, W. Nucleoside analogues: Molecular mechanisms signaling cell death. Oncogene 2008, 27, 6522–6537. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, F.; Amato, F.; Morgillo, C.M.C.M.; Pinto, B.; Santarpia, G.; Borbone, N.; D’Errico, S.; Catalanotti, B.; Piccialli, G.; Castaldo, G.; et al. Peptide nucleic acids as miRNA target protectors for the treatment of cystic fibrosis. Molecules 2017, 22, 1144. [Google Scholar] [CrossRef] [PubMed]
- Poppe, H.; Rybalkin, S.D.; Rehmann, H.; Hinds, T.R.; Tang, X.B.; Christensen, A.E.; Schwede, F.; Genieser, H.G.; Bos, J.L.; Doskeland, S.O.; et al. Cyclic nucleotide analogues as probes of signaling pathways. Nat. Methods 2008, 5, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.M.; Piazza, G.A.; Tinsley, H.N. The role of cyclic nucleotide signaling pathways in cancer: Targets for prevention and treatment. Cancers 2014, 6, 436–458. [Google Scholar] [CrossRef] [PubMed]
- Clapper, D.L.; Walseth, T.F.; Dargie, P.J.; Lee, H.C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987, 262, 9561–9568. [Google Scholar] [CrossRef]
- Guse, A.H. Calcium mobilizing second messengers derived from NAD. Biochim. Biophys. Acta 2015, 1854, 1132–1137. [Google Scholar] [CrossRef]
- Gul, R.; Park, J.H.; Kim, S.Y.; Jang, K.Y.; Chae, J.K.; Ko, J.K.; Kim, U.H. Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy. Cardiovasc. Res. 2009, 81, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.V.L.; Walseth, T.F. Medicinal chemistry and pharmacology of cyclic ADP-ribose. Curr. Mol. Med. 2004, 4, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Tsuzuki, T.; Murayama, T.; Kameda, T.; Kumaki, Y.; Sakurai, T.; Fukuda, H.; Watanabe, M.; Arisawa, M.; Shuto, S. Synthesis of 8-Substituted Analogues of Cyclic ADP-4-Thioribose and Their Unexpected Identification as Ca2+-Mobilizing Full Agonists. J. Med. Chem. 2017, 60, 5868–5875. [Google Scholar] [CrossRef]
- Watt, J.M.; Graeff, R.; Thomas, M.P.; Potter, B.V.L. Second messenger analogues highlight unexpected substrate sensitivity of CD38: Total synthesis of the hybrid “l-cyclic inosine 5′-diphosphate ribose”. Sci. Rep. 2017, 7, 16100. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, M.; Tsuzuki, T.; Takano, S.; Murayama, T.; Sakurai, T.; Kameda, T.; Fukuda, H.; Arisawa, M.; Shuto, S. Design, Synthesis, and Identification of 4″α-Azidoethyl-cyclic ADP-Carbocyclic-ribose as a Highly Potent Analogue of Cyclic ADP-Ribose, a Ca2+-Mobilizing Second Messenger. J. Med. Chem. 2016, 59, 7282–7286. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Mayol, L.; Oliviero, G.; Piccialli, G.; Varra, M. Synthesis of a novel N-1 carbocyclic, N-9 butyl analogue of cyclic ADP ribose (cADPR). Tetrahedron 2002, 58, 363–368. [Google Scholar] [CrossRef]
- Wagner, G.K.; Guse, A.H.; Potter, B.V.L. Rapid synthetic route toward structurally modified derivatives of cyclic adenosine 5’-diphosphate ribose. J. Org. Chem. 2005, 70, 4810–4819. [Google Scholar] [CrossRef] [PubMed]
- Mahal, A.; D’Errico, S.; Borbone, N.; Pinto, B.; Secondo, A.; Costantino, V.; Tedeschi, V.; Oliviero, G.; Piccialli, V.; Piccialli, G. Synthesis of cyclic N1-pentylinosine phosphate, a new structurally reduced cADPR analogue with calcium-mobilizing activity on PC12 cells. Beilstein J. Org. Chem. 2015, 11, 2689–2695. [Google Scholar] [CrossRef]
- D’Errico, S.; Borbone, N.; Catalanotti, B.; Secondo, A.; Petrozziello, T.; Piccialli, I.; Pannaccione, A.; Costantino, V.; Mayol, L.; Piccialli, G.; et al. Synthesis and Biological Evaluation of a New Structural Simplified Analogue of cADPR, a Calcium-Mobilizing Secondary Messenger Firstly Isolated from Sea Urchin Eggs. Mar. Drugs 2018, 16, 89. [Google Scholar] [CrossRef]
- D’Errico, S.; Basso, E.; Falanga, A.P.A.P.; Marzano, M.; Pozzan, T.; Piccialli, V.; Piccialli, G.; Oliviero, G.; Borbone, N. New linear precursors of cIDPR derivatives as stable analogues of cADPR: A potent second messenger with Ca2+-Modulating activity isolated from sea urchin eggs. Mar. Drugs 2019, 17, 476. [Google Scholar] [CrossRef]
- D’Errico, S.; Greco, F.; Patrizia Falanga, A.; Tedeschi, V.; Piccialli, I.; Marzano, M.; Terracciano, M.; Secondo, A.; Roviello, G.N.; Oliviero, G.; et al. Probing the Ca2+ mobilizing properties on primary cortical neurons of a new stable cADPR mimic. Bioorg. Chem. 2021, 117, 105401. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Piccialli, G.; Mayol, L. A solid-phase approach to the synthesis of N-1-alkyl analogues of cyclic inosine-diphosphate-ribose (cIDPR). Tetrahedron 2010, 66, 1931–1936. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-phase synthesis of a new diphosphate 5-aminoimidazole-4-carboxamide riboside (AICAR) derivative and studies toward cyclic AICAR diphosphate ribose. Molecules 2011, 16, 8110–8118. [Google Scholar] [CrossRef]
- Bonnac, L.F.; Dreis, C.D.; Geraghty, R.J. Structure activity relationship, 6-modified purine riboside analogues to activate hSTING, stimulator of interferon genes. Bioorgan. Med. Chem. Lett. 2019, 126819. [Google Scholar] [CrossRef] [PubMed]
- Hulpia, F.; Bouton, J.; Campagnaro, G.D.; Alfayez, I.A.; Mabille, D.; Maes, L.; de Koning, H.P.; Caljon, G.; Van Calenbergh, S. C6–O-alkylated 7-deazainosine nucleoside analogues: Discovery of potent and selective anti-sleeping sickness agents. Eur. J. Med. Chem. 2020, 188, 112018. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Oliviero, G.; Amato, J.; Borbone, N.; Cerullo, V.; Hemminki, A.; Piccialli, V.; Zaccaria, S.; Mayol, L.; Piccialli, G. Synthesis and biological evaluation of unprecedented ring-expanded nucleosides (RENs) containing the imidazo[4,5-d][1,2,6]oxadiazepine ring system. Chem. Commun. 2012, 48, 9310–9312. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Saleh, S.; Blair, I.A. Selective Deprotection of Silyl Ethers. Tetrahedron Lett. 1989, 30, 19–22. [Google Scholar] [CrossRef]
- Leškovskis, K.; Zaķis, J.M.; Novosjolova, I.; Turks, M. Applications of Purine Ring Opening in the Synthesis of Imidazole, Pyrimidine, and New Purine Derivatives. Eur. J. Org. Chem. 2021, 2021, 5027–5052. [Google Scholar] [CrossRef]
- Shuto, S.; Shirato, M.; Sumita, Y.; Ueno, Y.; Matsuda, A. Nucleosides and Nucleotides. 173. Synthesis of Cyclic IDP-carbocyclic-ribose, a Stable Mimic of Cyclic ADP-Ribose. Significant Facilitation of the Intramolecular Condensation Reaction of N-1-(Carbocyclic-ribosyl)inosine 5’’, 6’’-Diphosphate Derivatives by an 8-Bromo-Sustitution at the Hypoxanthine Moiety. J. Org. Chem. 1998, 63, 1986–1994. [Google Scholar]
- De Napoli, L.; Di Fabio, G.; Messere, A.; Montesarchio, D.; Piccialli, G.; Varra, M. Synthetic studies on the glycosylation of the base residues of inosine and uridine. J. Chem. Soc. Perkin Trans. 1999, 1, 3489–3493. [Google Scholar] [CrossRef]
- Hyde, R.M.; Broom, A.D.; Buckheit, R.W. Antiviral amphipathic oligo- and polyribonucleotides: Analogue development and biological studies. J. Med. Chem. 2003, 46, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Karnekanti, R.; Hanumaiah, M.; Sharma, G.V.M. Stereoselective Total Synthesis of (+)-Anamarine and 8-epi-(-)-Anamarine from D-Mannitol. Synthesis 2015, 47, 2997–3008. [Google Scholar] [CrossRef][Green Version]
- Lanier, M.L.; Park, H.; Mukherjee, P.; Timmerman, J.C.; Ribeiro, A.A.; Widenhoefer, R.A.; Hong, J. Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation. Chem.-A Eur. J. 2017, 23, 7180–7184. [Google Scholar] [CrossRef] [PubMed]
- Véliz, E.A.; Beal, P.A. 6-Bromopurine nucleosides as reagents for nucleoside analogue synthesis. J. Org. Chem. 2001, 66, 8592–8598. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, H.S.; Pauff, S.M.; Kosmidis, T.D.; Taladriz-Sender, A.; Rutherford, O.I.; Hatit, M.Z.C.; Fenner, S.; Watson, A.J.B.; Burley, G.A. Modular, Step-Efficient Palladium-Catalyzed Cross-Coupling Strategy to Access C6-Heteroaryl 2-Aminopurine Ribonucleosides. Org. Lett. 2017, 19, 3759–3762. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.J.; Shurtleff, V.W.; Johnson, A.M.; El Marrouni, A. Synthesis of C6-Substituted Purine Nucleoside Analogues via Late-Stage Photoredox/Nickel Dual Catalytic Cross-Coupling. ACS Med. Chem. Lett. 2021, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
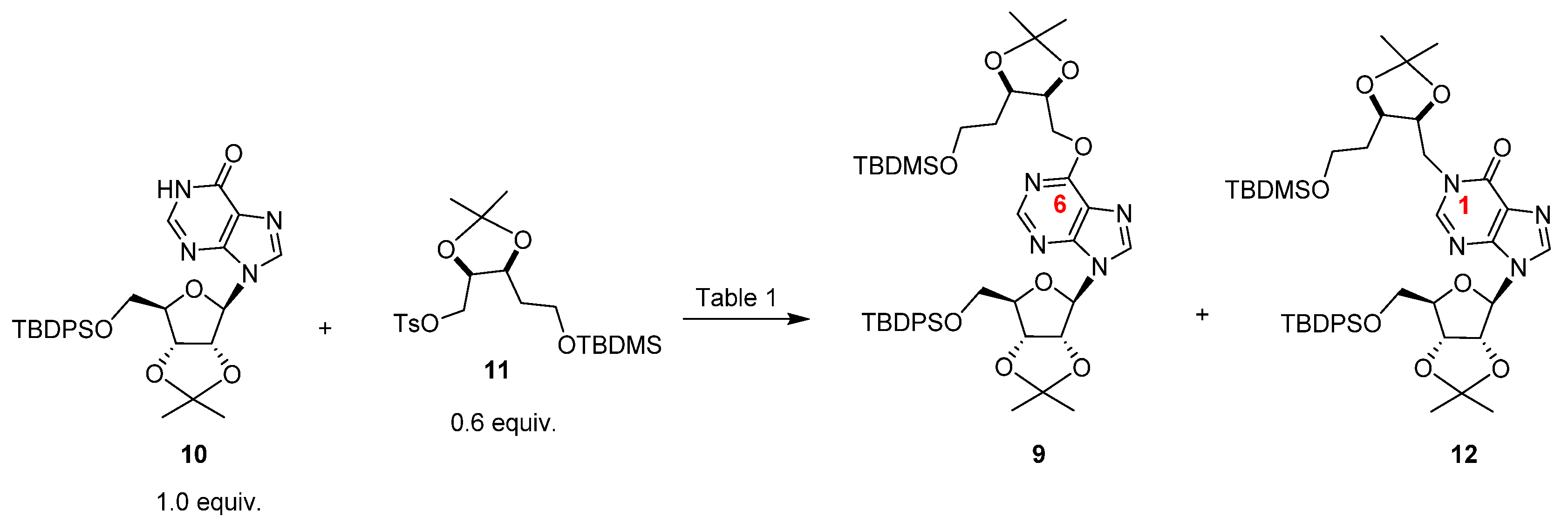
| Entry | Base | Equivalents 2 | Temperature (°C) | Yield (%) | 9:12 |
|---|---|---|---|---|---|
| 1 | K2CO3 | 1 or 3 | r.t. | No reaction | - |
| 2 | K2CO3 | 3 | 80 | 10 | 20:80 |
| 3 | Triethylamine | 1 or 3 | r.t. | No reaction | - |
| 4 | Triethylamine | 3 | 80 | No reaction | - |
| 5 | DBU | 1 or 3 | r.t. | No reaction | - |
| 6 | DBU | 3 | 80 | 70 | 30:70 |
| 7 | DBU | 3 | 120 | 30 | 60:40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzano, M.; Terracciano, M.; Piccialli, V.; Mahal, A.; Nilo, R.; D’Errico, S. O6-[(2″,3″-O-Isopropylidene-5″-O-tbutyldimethylsilyl)pentyl]-5′-O-tbutyldiphenylsilyl-2′,3′-O-isopropylideneinosine. Molbank 2022, 2022, M1345. https://doi.org/10.3390/M1345
Marzano M, Terracciano M, Piccialli V, Mahal A, Nilo R, D’Errico S. O6-[(2″,3″-O-Isopropylidene-5″-O-tbutyldimethylsilyl)pentyl]-5′-O-tbutyldiphenylsilyl-2′,3′-O-isopropylideneinosine. Molbank. 2022; 2022(1):M1345. https://doi.org/10.3390/M1345
Chicago/Turabian StyleMarzano, Maria, Monica Terracciano, Vincenzo Piccialli, Ahmed Mahal, Roberto Nilo, and Stefano D’Errico. 2022. "O6-[(2″,3″-O-Isopropylidene-5″-O-tbutyldimethylsilyl)pentyl]-5′-O-tbutyldiphenylsilyl-2′,3′-O-isopropylideneinosine" Molbank 2022, no. 1: M1345. https://doi.org/10.3390/M1345
APA StyleMarzano, M., Terracciano, M., Piccialli, V., Mahal, A., Nilo, R., & D’Errico, S. (2022). O6-[(2″,3″-O-Isopropylidene-5″-O-tbutyldimethylsilyl)pentyl]-5′-O-tbutyldiphenylsilyl-2′,3′-O-isopropylideneinosine. Molbank, 2022(1), M1345. https://doi.org/10.3390/M1345