Charge-Transfer Adducts of Chalcogenourea Derivatives of N-Heterocyclic Carbenes with Iodine Monochloride
Abstract
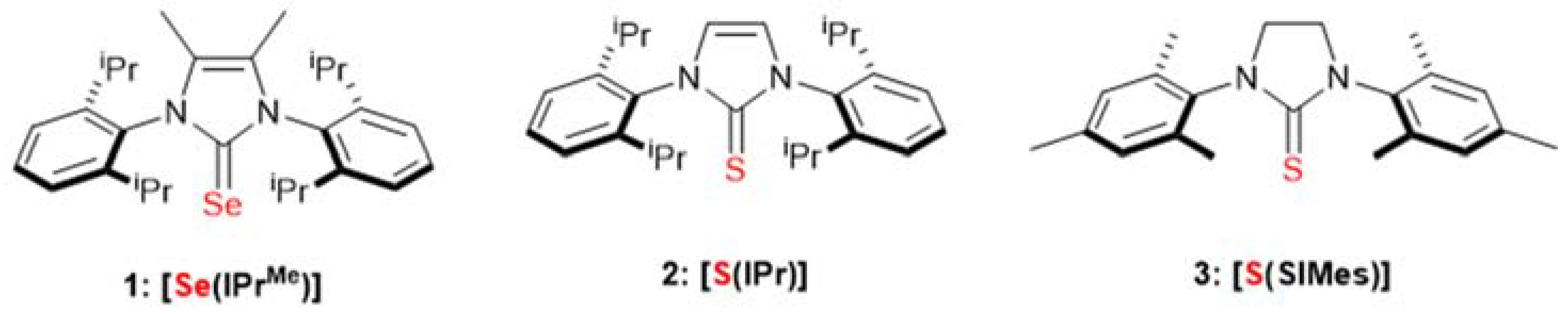
1. Introduction
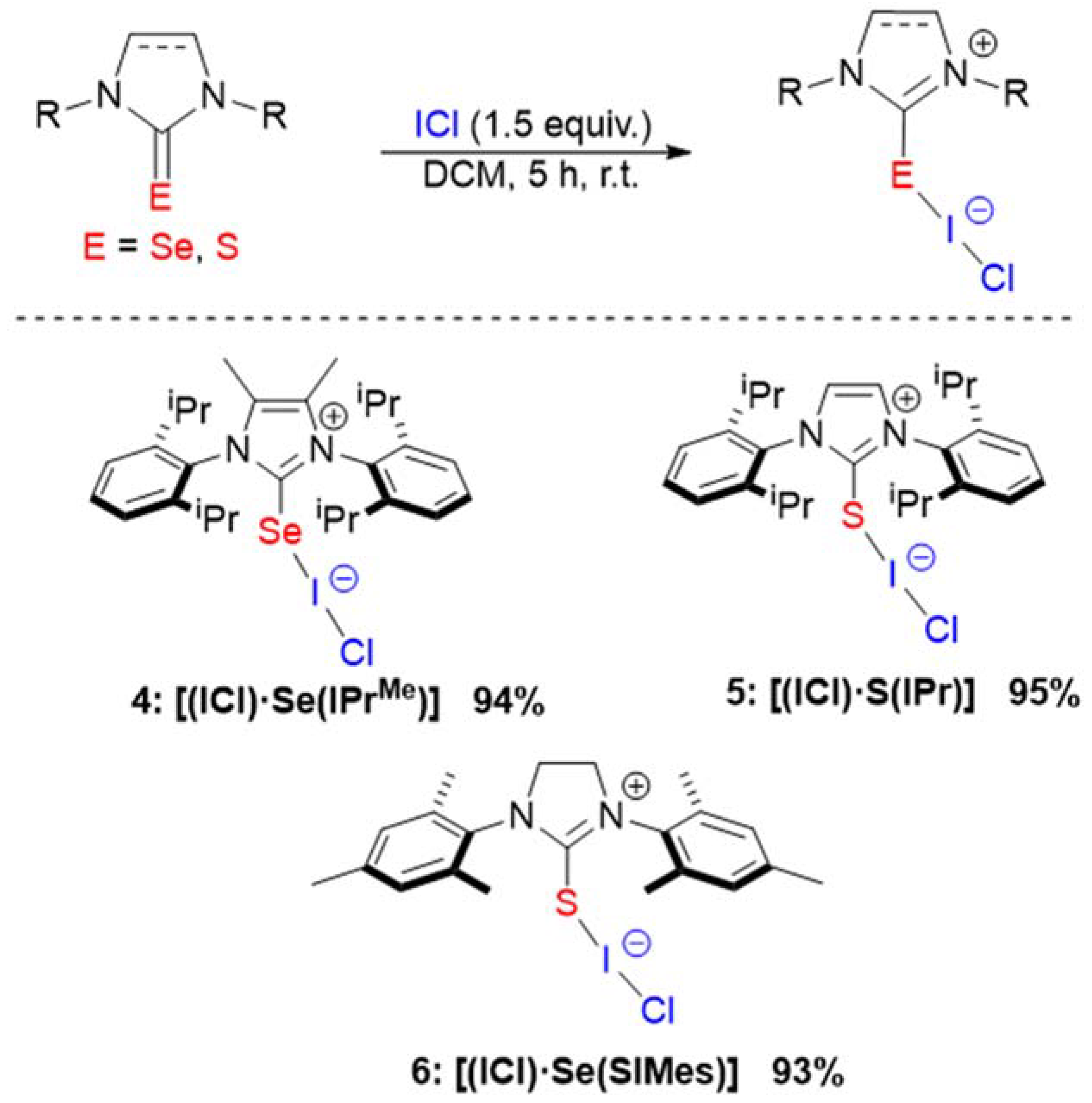
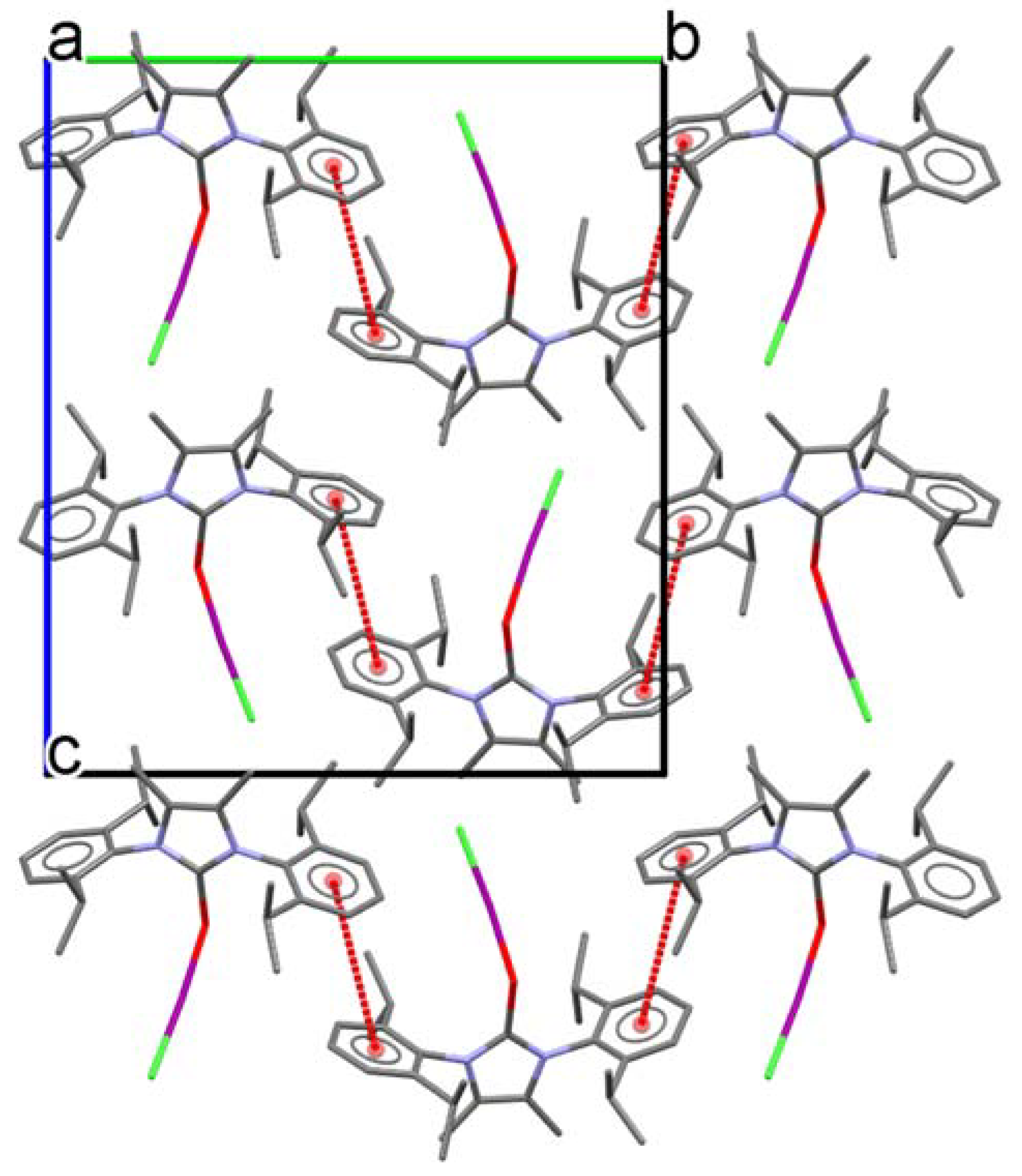
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molter, A.; Mohr, F. Gold complexes containing organoselenium and organotellurium ligands. Coord. Chem. Rev. 2010, 254, 19–45. [Google Scholar] [CrossRef]
- Doddi, A.; Peters, M.; Tamm, M. N-Heterocyclic Carbene Adducts of Main Group Elements and Their Use as Ligands in Transition Metal Chemistry. Chem. Rev. 2019, 119, 6994–7112. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Shah, A. Synthesis and biological applications of selenoureas. Appl. Organometal. Chem. 2014, 28, 61–73. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea Derivatives in Drug Design and Medicinal Chemistry: A Short Review. J. Drug Des. Med. Chem. 2016, 2, 10–20. [Google Scholar] [CrossRef]
- Manna, D.; Roy, G.; Mugesh, G. Antithyroid Drugs and Their Analogues: Synthesis, Structure, and Mechanism of Action. Acc. Chem. Res. 2013, 46, 2706–2715. [Google Scholar] [CrossRef]
- Mancini, A.; Aragoni, M.C.; Bricklebank, N.; Castellano, C.; Demartin, F.; Isaia, F.; Lippolis, V.; Pintus, A.; Arca, M. Formation of T-shaped versus Charge-Transfer Molecular Adducts in the Reactions Between Bis(thiocarbonyl) Donors and Br2 and I2. Chem. Asian J. 2013, 8, 639–649. [Google Scholar] [CrossRef]
- Isaia, F.; Aragoni, M.C.; Arca, M.; Caltagirone, C.; Demartin, F.; Garau, A.; Lippolis, V. Gold oxidative dissolution by (thioamide)–I2 adducts. Dalton Trans. 2013, 42, 492–498. [Google Scholar] [CrossRef]
- Boyle, P.D.; Godfrey, S.M. The reactions of sulfur and selenium donor molecules with dihalogens and interhalogens. Coord. Chem. Rev. 2001, 223, 265–299. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Verani, G. Charge-transfer adducts between donors containing chalcogens (S and Se) and di-iodine: Solution studies. Coord. Chem. Rev. 1999, 184, 271–290. [Google Scholar] [CrossRef]
- Cristiani, F.; Devillanova, F.; Isaia, F.; Lippolis, V.; Verani, F. Charge transfer complexes of benzoxazole-2(3H)-thione and benzoxazole-2(3H)-selone with diiodine: X-ray crystal structure of benzoxazole-2(3H)-thione bis(diiodine). Polyhedron 1995, 14, 2937–2943. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Demartin, F.; Devillanova, F.; Garau, A.; Isaia, F.; Lelj, F.; Lippolis, V.; Verani, G. Mechanistic Aspects of the Reaction between Br2 and Chalcogenone Donors (LE.; E=S, Se): Competitive Formation of 10-E-3, T-Shaped 1:1 Molecular Adducts, Charge-Transfer Adducts, and [(LE)2]2+ Dications. Chem. Eur. J. 2001, 7, 3122–3133. [Google Scholar] [CrossRef]
- Demartin, F.; Deplano, P.; Devillanova, F.A.; Isaia, F.; Lippolis, V.; Verani, G. Conductivity, FT-Raman spectra, and x-ray crystal structures of two novel [D2I]In (n = 3 and D = N-methylbenzothiazole-2(3H)-selone; n = 7 and D = N-methylbenzothiazole-2(3H)-thione) iodonium salts. First example of I-.3I2 heptaiodide. Inorg. Chem. 1993, 32, 3694–3699. [Google Scholar] [CrossRef]
- Demartin, F.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Verani, G. Reactions of N-methylbenzothiazole-2(3H)-thione (1) and -selone (2) with ICl: Synthesis and X-ray crystal structures of the charge-transfer adducts 1·ICl (I) and 2·ICl (II). Polyhedron 1999, 18, 3107–3113. [Google Scholar] [CrossRef]
- Nosco, D.L.; Heeg, M.J.; Glick, M.D.; Elder, R.C.; Deutsch, E. Coordination stabilization of organic intermediates. Crystal structure of {[(en)2Co(SCH2CH2NH2)]2I}(NO3)5.4H2O, a stable complex of iodine(I). J. Am. Chem. Soc. 1980, 102, 7784–7786. [Google Scholar]
- Daga, V.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Santos, J.H.Z.D.; Butler, I.S. Synthesis, spectroscopic and structural characterization of novel diiodine adducts with the heterocyclic thioamides, thiazolidine-2-thione (tzdtH), benzothiazole-2-thione (bztzdtH) and benzimidazole-2-thione (bzimtH). Eur. J. Inorg. Chem. 2002, 2002, 1718. [Google Scholar] [CrossRef]
- Antoniadis, C.D.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Butler, I.S. Synthesis, X-ray Characterisation and Studies of the New Ionic Complex [Bis(pyridin-2-yl) disulfide] Triiodide, Obtained by Oxidation of 2-Mercaptopyridine with I2-Implications in the Mechanism of Action of Antithyroid Drugs. Eur. J. Inorg. Chem. 2004, 2004, 4324–4329. [Google Scholar] [CrossRef]
- Antoniadis, C.D.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Butler, I.S. Synthesis, X-ray characterization and study of new ionic complexes of 2-pyridone, obtained by oxidation with I2. New J. Chem. 2005, 29, 714–720. [Google Scholar] [CrossRef]
- Corban, G.J.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Tiekink, E.R.T.; Butler, S.; Drougas, E.; Kosmas, A.M. Synthesis, Structural Characterization, and Computational Studies of Novel Diiodine Adducts with the Heterocyclic Thioamides N-methylbenzothiazole-2-thione and benzimidazole-2-thione: Implications with the Mechanism of Action of Antithyroid Drugs. Inorg. Chem. 2005, 44, 8617–8627. [Google Scholar] [CrossRef]
- Williams, D.J.; Vanderveer, D.; Crouse, B.R.; Raye, R.R.; Carter, T.; Hagen, K.S.; Brewer, M. Spectroscopic properties and molecular structure of 1,3-dimethyl-2-(Se, Se-dibromose1eno)-2(3H)-imidazolylidene. Main Group Chem. 1997, 2, 61–66. [Google Scholar] [CrossRef]
- Pérez, E.J.J.; Aragoni, M.C.; Arca, M.; Blake, A.J.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Núñez, R.; Pintus, A.; et al. A Unique Case of Oxidative Addition of Interhalogens IX (X=Cl, Br) to Organodiselone Ligands: Nature of the Chemical Bonding in Asymmetric I-Se-X Polarised Hypervalent Systems. Chem. Eur. J. 2011, 17, 11497–11514. [Google Scholar] [CrossRef]
- Saab, M.; Nelson, D.J.; Tzouras, N.V.; Bayrakdar, T.A.C.A.; Nolan, S.P.; Nahra, F.; Hecke, K.V. Straightforward access to chalcogenoureas derived from N-heterocyclic carbenes and their coordination chemistry. Dalton Trans. 2020, 49, 12068–12081. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.F. Structural Inorganic Chemistry, 5th ed.; Clarendon Press: Oxford, UK, 1984. [Google Scholar]
- Srinivas, K.; Suresh, P.; Babu, C.N.; Sathyanarayana, A.; Prabusankar, G. Heavier chalcogenone complexes of bismuth(III) trihalides: Potential catalysts for acylative cleavage of cyclic ethers. RSC Adv. 2015, 5, 15579–15590. [Google Scholar] [CrossRef]
- Paas, M.; Wibbeling, B.; Fröhlich, R.; Hahn, F.E. Silver and Rhodium Complexes of Stable, Monomeric Imidazolidin-2-ylidenes: Synthesis, Reactivity and Decomposition Pathway. Eur. J. Inorg. Chem. 2006, 158–162. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System; Rigaku Corporation: Oxford, UK, 2019. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
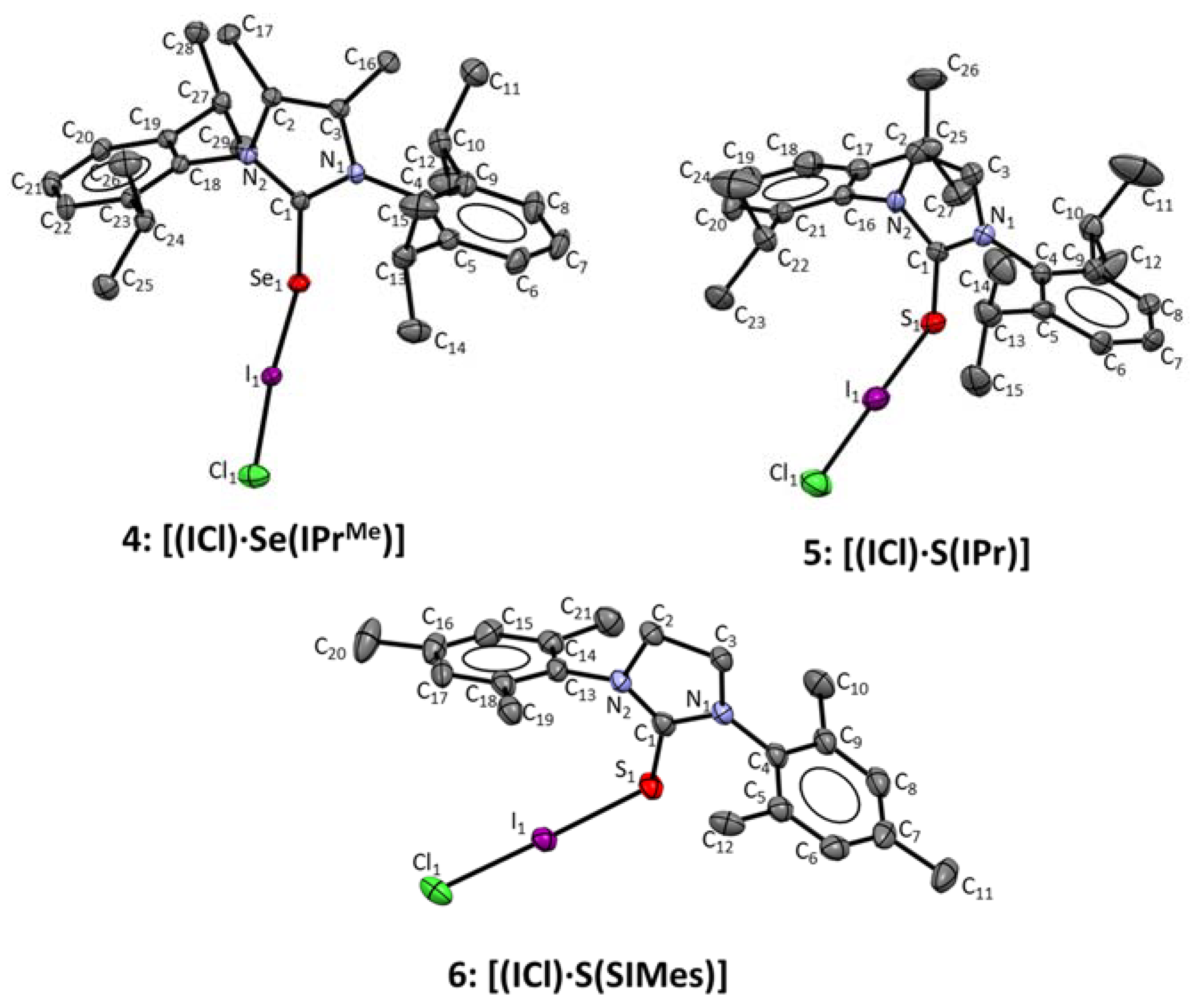
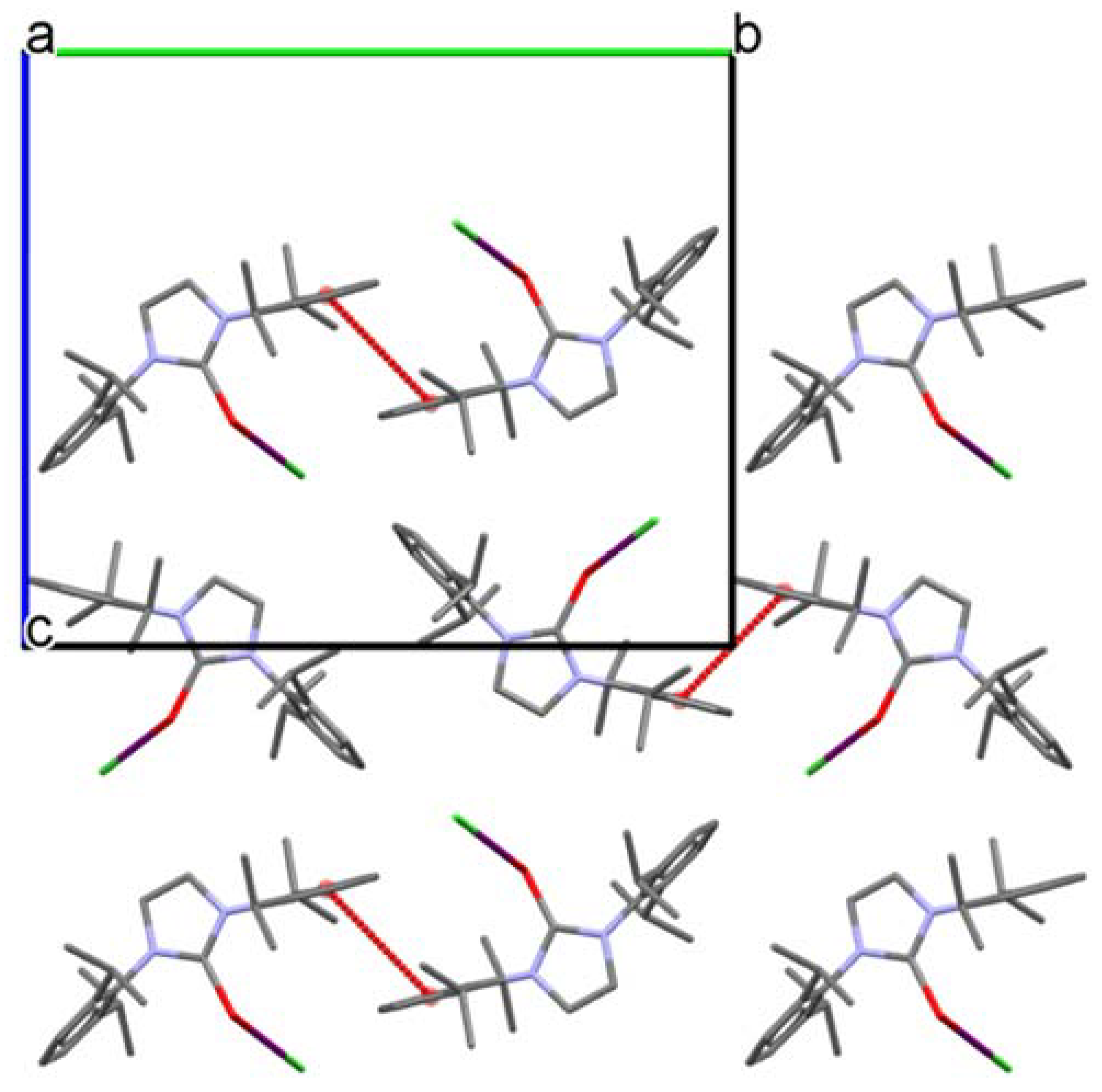
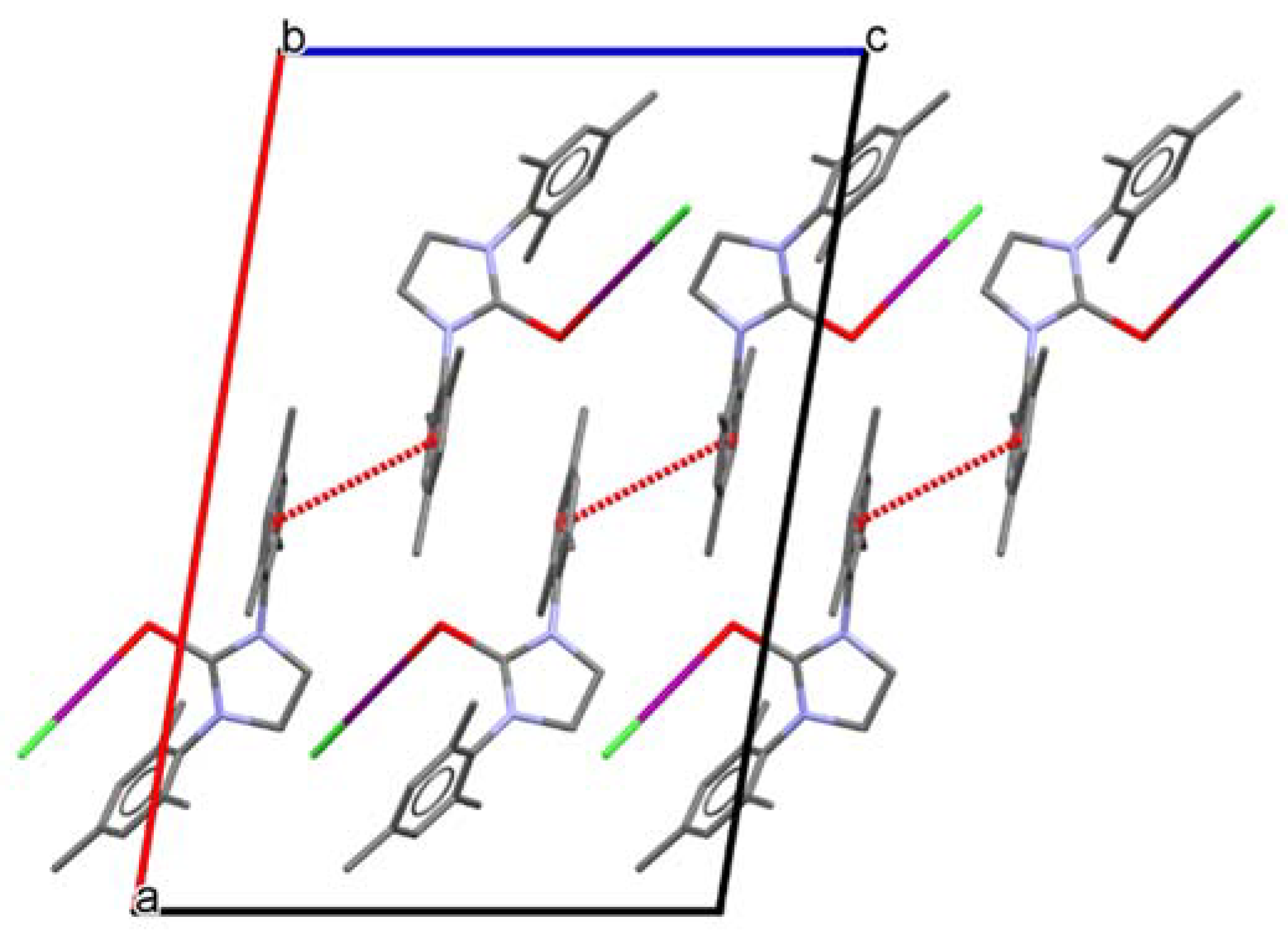
| Structure | C1-E1 | E1-I1 | I1-Cl1 | C1-E1-I1 | E-I1-Cl1 |
|---|---|---|---|---|---|
| [(ICl)·Se(IPrMe)] (4) | 1.880(2) | 2.6516(3) | 2.6903(7) | 101.63(8) | 175.143(18) |
| [(ICl)·S(IPr)] (5) | 1.726(7) | 2.545(2) | 2.742(2) | 101.3(2) | 178.50(6) |
| [(ICl)·S(SIMes)] (6) | 1.728(4) | 2.6034(11) | 2.5970(13) | 103.91(15) | 178.71(4) |
| [Se(IPrMe)] a (1) | 1.827(5) | - | - | - | - |
| [S(IPr)] b (2) | 1.670(3) | - | - | - | - |
| [S(SIMes)] c (3) | 1.666(2) | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saab, M.; Nahra, F.; Van Hecke, K. Charge-Transfer Adducts of Chalcogenourea Derivatives of N-Heterocyclic Carbenes with Iodine Monochloride. Molbank 2022, 2022, M1344. https://doi.org/10.3390/M1344
Saab M, Nahra F, Van Hecke K. Charge-Transfer Adducts of Chalcogenourea Derivatives of N-Heterocyclic Carbenes with Iodine Monochloride. Molbank. 2022; 2022(1):M1344. https://doi.org/10.3390/M1344
Chicago/Turabian StyleSaab, Marina, Fady Nahra, and Kristof Van Hecke. 2022. "Charge-Transfer Adducts of Chalcogenourea Derivatives of N-Heterocyclic Carbenes with Iodine Monochloride" Molbank 2022, no. 1: M1344. https://doi.org/10.3390/M1344
APA StyleSaab, M., Nahra, F., & Van Hecke, K. (2022). Charge-Transfer Adducts of Chalcogenourea Derivatives of N-Heterocyclic Carbenes with Iodine Monochloride. Molbank, 2022(1), M1344. https://doi.org/10.3390/M1344








