Abstract
Nitroimidazoles are pharmacophoric groups responsible for important antiparasitic activity against several infectious diseases. 2-Nitroimidazoles are found in some antiparasitic drugs and are one of the main moieties responsible for the biological activities exhibited. As an example, we can mention the drug benznidazole, the only drug available in Brazil for the treatment of Chagas disease. This work describes an efficient methodology for the synthesis of 2-nitro-1-vinyl-1H-imidazole through a simple and direct approach, as well as its full characterization and biological assessment. The antiparasitic evaluation of 2-nitro-1-vinyl-1H-imidazole against Trypanosoma cruzi (Tulahuen C2C4-LacZ strain) showed IC50 = 4.8 μM on amastigotes and low cytotoxicity against LLC-MK2 cells (IC50 > 500 μM), validating 2-nitro-1-vinyl-1H-imidazole as a biologically active structural subunit for anti-T. cruzi activity. The results presented herein demonstrate that 2-nitro-1-vinyl-1H-imidazole can be easily obtained, possessing great potential for use in the design of new antichagasic drugs through a molecular hybridization strategy using known coupling reactions.
1. Introduction
Nitroimidazoles are a class of molecules that present in their structure a five-membered heterocycle, with two nitrogen atoms in the 1 and 3 positions and a nitro group that can appear in the 2, 4 or 5 position. The first nitroimidazole isolated and characterized from a natural source was azomycin 1 (2-nitroimidazole), a natural antibiotic from Streptomyces eurocidicus, in the 1950s, while a group of scientists analyzed the activity of this extract against Trichomonas vaginalis [1,2]. 2-Nitro-1-vinyl-1H-imidazole 2 was first described in the 1984 Hoffman-La Roche patent as an antiparasitic agent with filarcidal activity, exhibiting average ED90 values against Litomosoides sigmodontis, formerly known as L. carinii, in cotton rats at doses of 12 mg/kg [3,4]. Despite its description in this patent, which, as far as we know, is the only description of derivative 2 in the literature, the work does not describe characterization data or even further details on the methodology used in its preparation. The use of nitroimidazoles as antiparasitic agents deserves significant attention, since important drugs currently used to treat both parasitic and bacterial infections have a nitroheterocycle group in their structure, such as benznidazole 3 (2-nitroimidazole) and nifurtimox 4 (5-nitrofuran). These are the only drugs used to treat Chagas disease that possess protozoa hemoflagellate T. cruzi as an etiologic agent [5,6,7], in addition to megazole 5 and fexinidazol 6 (both 1-methyl-5-nitroimidazole), which have great antichagasic potential [8], and metronidazole 7 (2-methyl-5-nitroimidazole) for the treatment of infections caused by protozoa such as Trichomonas vaginalis [1], Giardia Lamblia [9] and bacterial infections. The nitroheterocyclic portion is pharmacophoric to the drugs shown in Figure 1, characterizing this core as a fundamental subunit in the structure of this set of drugs.
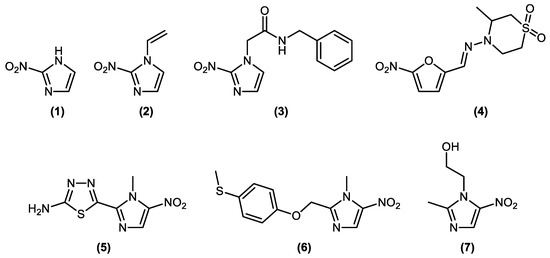
Figure 1.
Drugs and other bioactive molecules containing the nitroimidazole core.
The objective of this work is the synthesis, full characterization, and anti-T. cruzi in vitro evaluation of 2-nitro-1-vinyl-1H-imidazole 2 (Scheme 1). This molecule, in addition to its potential anti-T. cruzi activity, is also a useful building block in molecular hybridization strategies applied to the design of new antiparasitic drugs, enabling the homologation of the 2-nitrimidazole pharmacophore to the structure of different natural products, or even other known antiparasitic agents. This type of approach has the ability to lead to structures with enhanced antiparasitic profiles when compared to their precursors [10,11]. The attachment of an alkene moiety to N1 of the 2-nitroimidazole core enables its linkage to other bioactive molecules through known C–C coupling reactions [12]. To validate the use of this structural subunit in the development of new antichagasic drugs, assays against amastigotes of T. cruzi were carried out, as well as a cytotoxicity assay on LLC-MK2 (host cells) of 2-nitro-1-vinyl-1H-imidazole 2, as well as its precursor, the natural antibiotic azomycin 1 (Scheme 1).
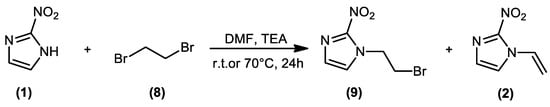
Scheme 1.
Reaction of 2-nitroimidazole and 1,2-dibromoethane.
2. Results and Discussion
2.1. Chemistry
Interest in the synthesis of imidazole derivatives is mainly due to their therapeutic properties against several infectious agents [13]. A technique widely used in medicinal chemistry is molecular hybridization. Anchoring between pharmacophores requires the occurrence of chemically labile groups that can be easily and selectively modified. Our original objective in this work was the synthesis of the alkylant 1-(2-bromoethyl)-2-nitro-1H-imidazole (9). We thus reacted 2-nitroimidazole (1) with excess 1,2-dibromoethane (8) at room temperature (Scheme 1). It was observed, however, the formation of two products, which were identified as the desired product and 2-nitro-1-vinyl-1H-imidazole (2). The synthesis of 2-nitro-1-vinyl-1H-imidazole has been previously described in the literature [3]. In this new protocol, the main advantage is the possibility of obtaining halogenated or vinylic derivatives, simply by changing reaction conditions such as temperature and stoichiometry.
The protocol was optimized to enhance yield and conversion to the vinyl derivative. The reaction was carried out under heating (70 °C), using different stoichiometric proportions of 1,2-dibromoethane (3–10 mmol equivalent) relative to 2-nitroimidazole. The highest conversion (close to 100%) to the vinyl derivative and yield (52%) were observed at the proportion of 4.0 mmol equivalent of 1,2-dibromoethane The formation of 1,2-bis(2-nitro-1H-imidazol-1-yl)ethane, as a by-product, in trace amounts was observed by LCMS analysis of the isolated product (m/z 253.0 [M + H]+, calculated for C8H8N6O4+, 253.0) (Figure S6). Mechanistically, our proposal is that part of the 2-nitroimidazolide ion (A) acts as a nucleophile, attacking 1,2-dibromoethane in an SN2 fashion, while the remaining part of the ion (A) acts as a base, causing E2-type dehydrohalogenation of the bromoalkylated intermediate. The proposed reaction mechanism is presented in Scheme 2.
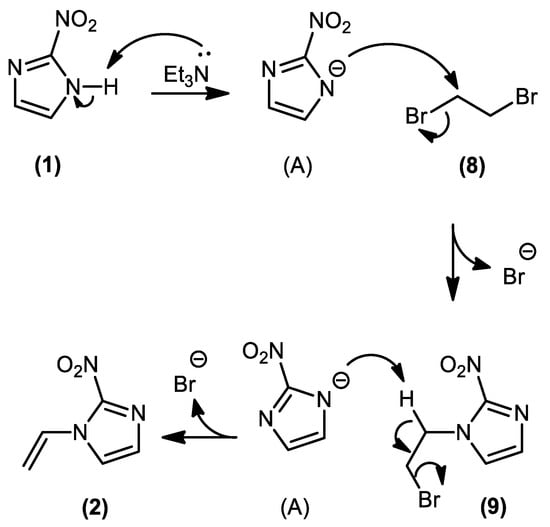
Scheme 2.
Proposed reaction mechanism for the selective synthesis of 2-nitro-1-vinyl-1H-imidazole (4).
2.2. Biological Assays
Compounds 1 and 2 had their cytotoxicity profiles evaluated against LLC-MK2 cells, as well as their antiparasitic activity against amastigotes of T. cruzi (Tulahuen C2C4 LacZ strain) [14,15]. These molecules showed an excellent cytotoxicity profile, with emphasis on the vinyl molecule that presented low cytotoxicity with an IC50 above 500 µM against LLC-MK2 cells. In addition to showing promising activity against amastigotes of T. cruzi with an IC50 = 4.76 µM for 2-nitro-1-vinyl-1H-imidazole 2 and an IC50 = 5.74 µM for its precursor, azomycin 1, these data are shown in Table 1. To the best of our knowledge, this is the first evaluation of the natural antibiotic azomycin 1 against Trypanosoma cruzi. These results highlight 2-nitro-1-vinyl-1H-imidazole as a building block in a molecular hybridization strategy through a coupling reaction involving an alkene moiety, leading to the formation of carbon–carbon bonds. The insertion of this group can improve antiparasitic activity, enhancing the biological activity profile of a series of molecules. The molecular hybridization strategy has been used in several works now in progress by our research group, providing new hybrids with very promising antiparasitic activities.

Table 1.
Results of biological activity for 2-nitro-1-vinyl-1H-imidazole and its precursor azomycin.
3. Materials and Methods
3.1. Chemistry
All chemical reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless otherwise stated. Solvents were treated with activated molecular sieves (3A) prior to use. Reactions were monitored by thin-layer chromatography (TLC) on 0.25 mm Merck (Darmstadt, Germany) silica gel plates (60F-254) and visualized under a UV lamp (254 and 365 nm). All melting points (mp) were measured using a Melting Point AAKER model PFM-II and were uncorrected. 1H-NMR and 13C-NMR spectra were measured on a BRUKER Ultrashield Plus spectrometer (Billerica, MA, USA) at 25 °C and referenced to TMS. Chemical shifts are reported in ppm (δ) using the residual solvent line as an internal standard. Splitting patterns are designed as s, singlet; d, doublet; t, triplet; m, multiplet; brs, broad singlet. IR spectrum was recorded on a BRUKER-VERTEX 70 FT-IR spectrophotometer using an ATR apparatus. The liquid chromatography–mass spectrometry (LCMS) analyses were carried out on a Shimadzu LCMS 2020 (Shimadzu Inc., Kyoto, Japan). Analytical conditions: column: Kromasil C18, 150 mm × 4.6 mm × 5 µm (AkzoNobel, Amsterdam, the Netherland); mobile phase: water with 0.1% formic acid (A), acetonitrile with 0.1% formic acid (B), 1.0 mL/min, linear gradient (indicated on trace); injection volume: 10 µL; detectors: PDA (200–400 nm), ESI+ (low resolution).
2-Nitroimidazole (1) (200.0 mg, 1.77 mmol), 1,2-dibromoethane (4 mmol equivalent) and TEA (5 mmol equivalent) were dissolved in DMF (2 mL) in a round bottom flask. The reaction kept under stirring at 70 °C for 24 h. After complete consumption of the 1, monitored by analytical TLC (hexanes: DCM: ethyl acetate 5:2:3), it was added 30 mL cold 10% NaCl(aq) and then extracted with 3 × 20 mL ethyl acetate. The pooled organic phase was then washed with 5 × 20 mL 10% NH4Cl(aq) and 2 × 20 mL 10% NaHCO3(aq), dried over anhydrous sodium sulfate (Na2SO4), filtered, and the solvent removed in a rotary evaporator. The product was isolated as yellow crystals with 128.1 mg (0.92 mmoL, 52% yield). Mp = 62–64°. Structure was confirmed by 1H-NMR, 13C-NMR, IR, and MS: 1H-NMR (500 MHz, CDCl3): δ 7.63 (dd, J = 15.4, 8.5 Hz, 1H); 7.36 (s, 1H); 7.20 (s, 1H); 5.50 (dd, J = 15.4, 0.9 Hz, 1H); 5.31 (dd, J = 8.5, 0.9 Hz, 1H). 13 C-NMR (125 MHz, CDCl3): δ 130.45; 129.23; 122.41; 109.71. IR (ATR): 3155, 3128, 1635, 1527, 1348, 1265, 962, 835, 800 cm−1. LRMS, m/z 140.0 [M + H]+ (calculated for C5H6N3O2+, 140.0). All spectra obtained from product 2 are available in the supplementary material (Figures S1–S4). The purity of the final product was determined by RP-HPLC (Figure S5) and was >93%.
3.2. Biological Assays
Mammalian lineage cells were used to assess the cytotoxicity of the compounds tested. LLC-MK2 cells (ATCC®) were cultivated in DMEM (Dulbecco’s modified Eagle’s medium) + 5% FBS and incubated at 37 °C (5% CO2), with successive passages every 4–5 days. Cells were dissociated from the monolayer by treatment with a solution containing 0.25% w/v trypsin and 0.04% EDTA.
The antiparasitic activity assays used amastigotes of Tulahuen C2C4-LacZ strain of Trypanosoma cruzi. Amastigotes and trypomastigotes forms were cultivated by successive reinfections in LLC-MK2 cell monolayers in Dulbecco’s modified Eagle’s medium (DMEM) + 2% FBS and incubated at 37 °C (5% CO2). Trypomastigotes were collected from the culture supernatant between the 5th and 10th days after infection and separated from non-adhered cells by differential centrifugation.
3.2.1. Evaluation of Trypanocidal Activity against T. cruzi Amastigotes
In a flat 96-well plate, a suspension of 1 × 104 LLC-MK2 cells (ATCC) in DMEM (Dulbecco’s modified Eagle’s medium) + 2% FBS was added. Cells were incubated at 37 °C (5% CO2) for adhesion for 4 h and then washed with PBS to remove non-adhered cells. A suspension containing 1.5 × 105 T. cruzi trypomastigote forms of the Tulahuen C2C4 LacZ strain was added to the cells, followed by incubation at 37 °C (5% CO2) for 20 h to establish the infection. Noninternalized parasites were removed by three successive washes with PBS, followed by treatment with serial dilutions of compounds 1 and 2 in triplicate and predilution in DMEM + 2% FBS. Untreated, vehicle (0.2% v/v DMSO) and blank (no parasite addition) controls were included in the experiment. Benznidazole was used, in serial dilution, as a positive control. After incubation for 5 days (120 h), 30 µL of a 0.5 mM solution of chlorophenol red β-galactopyranoside substrate (CPRG) in PBS was then added with 0.9% v/v Igepal CA-630. After incubation for 1.5 h, absorbance was measured at λ = 570 nm with the aid of a plate reader.
3.2.2. Evaluation of Cytotoxicity against LLC-MK2 Cells
In a flat 96-well plate, a suspension of 1 × 104 LLC-MK2 cells (ATCC) in DMEM (Dulbecco’s modified Eagle’s medium) + 2% FBS was added. Cells were incubated at 37 °C (5% CO2) for 20 h and then washed with PBS to remove non-adhered cells. Cells were treated with serial dilutions of compounds 1 and 2 in triplicate and prediluted in DMEM + 2% FBS. Untreated, vehicle (0.2% v/v DMSO) and blank (no cell added) controls were included in the experiment. After incubation for 120 h, the supernatant was removed, the cell monolayer was washed with PBS, and the culture medium was renewed. Then, 20 µL of 3.0 mM MTT salt solution was added, followed by incubation for an additional 1.5 h. The supernatant was then removed, and the MTT formazan crystals were dissolved by the addition of 120 µL/well DMSO. After incubation for 1.5 h to dissolve the MTT crystals in the dark and at 37 °C, the absorbance was measured at λ = 570 nm with the aid of a plate reader.
4. Conclusions
The synthetic methodology described herein allowed the selective preparation of the 2-nitro-1-vinyl-1H-imidazole derivative 2 in satisfactory yield and high purity. The product was characterized through usual organic analysis methods and assessed against Trypanosoma cruzi amastigotes, showing a high toxic effect comparable to the reference drug, benznidazole 3. The synthetic precursor azomycin 1 was also evaluated against intracellular forms of the parasite, which, as far as we know, is the first description of the activity of this natural antibiotic against T. cruzi.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: 1H-NMR spectrum of compound 2 in CDCl3; Figure S2: 13C-NMR spectrum of compound 2 in CDCl3; Figure S3: IR spectrum of compound 2; Figure S4: ESI(+)-MS of compound 2; Figure S5: HPLC-PDA of compound 2; Figure S6: ESI(+)-MS of compound 1,2-bis(2-nitro-1H-imidazol-1-yl)ethane.
Author Contributions
Conceptualization: M.E.F.d.L. and D.D.-R. Synthesis and molecular characterization: G.A.d.S., P.P.-S. and M.E.F.d.L. Biological assessments: G.A.d.S., A.S.M.M.V., P.P.-S., D.C.d.A.P. and D.D.-R. Writing—original draft preparation: G.A.d.S., A.S.M.M.V., D.C.d.A.P. and M.E.F.d.L. Writing—review and editing: M.E.F.d.L. and D.D.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, financing code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Rosane N. Castro from Institute of Chemistry at Universidade Federal Rural do Rio de Janeiro (UFRRJ, Brazil) for the analytical facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cosar, C.; Julou, L. The activity of 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole (R. P. 8823) against experimental Trichomonas vaginalis infections. Ann. De L’institut Pasteur. 1959, 96, 238–241. [Google Scholar]
- Muller, C.E. ChemInform Abstract: Basic Chemistry of 2-Nitroimidazoles (Azomycin Derivatives). ChemInform 2000, 31, 47–59. [Google Scholar] [CrossRef]
- Bottmingen, W.H.; Binningen, H.S. Filaricidal 2-nitroimidazoles. U.S. Patent 4,456,610, 26 June 1984. [Google Scholar]
- Sgambatti De Andrade, S.; De Oliveira, R.M.; Almeida E Silva, S.; De Andrade, S.S.; Medicine, T.; Lucia, A.; Zicker, F.; Mauricio De Oliveira, R.; Almeida E Silva, S.; Luquetti, A.; et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infec-tion. Lancet 1996, 348, 1407–1413. Available online: https://pubmed.ncbi.nlm.nih.gov/8937280/ (accessed on 23 December 2021). [CrossRef]
- Mathew, N.; Kalyanasundaram, M. Antifilarial agents. Expert Opin. Ther. Pat. 2007, 17, 767–789. [Google Scholar] [CrossRef]
- Kratz, J.M.; Bournissen, F.G.; Forsyth, C.J.; Sosa-Estani, S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev. Clin. Pharmacol. 2018, 11, 943–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Pérez-Silanes, S. The role of imidazole and benzimidazole heterocycles in Cha-gas disease: A review. Eur. J. Med. Chem. 2020, 206, 112692. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Schlosser, S.; Burgess, A.; Duchêne, M. Nitroimidazole drugs vary in their mode of action in the human para-site Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 166–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas-Junior, C.; Barreiro, E.J.; Manssour, C.A.F. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S. Heck reaction—State of the art. Catalysts 2017, 7, 267. [Google Scholar] [CrossRef]
- Ang, C.W.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A. Nitroimidazoles: Molecular fireworks that combat a broad spec-trum of infectious diseases. J. Med. Chem. 2017, 60, 7636–7657. Available online: https://pubmed.ncbi.nlm.nih.gov/28463485/ (accessed on 23 December 2021). [CrossRef] [PubMed]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeber, F.; Boothroyd, J.C. Escherichia coli β-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene 1996, 169, 39–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).