Abstract
Herein, two title compounds, N-benzoyl-N′-(4′-cyanophenyl)thiourea (1) and N-(4-nitrobenzoyl)-N′-(4′-cyanophenyl)thiourea (2) were synthesized in a high yield, via different applications of aroyl isocyanate and 4-aminobenzonitrile. The structure of the prepared compounds was characterized by elemental analysis and FT-IR, 1H, and 13C-NMR spectroscopic methods. The crystal structure of the title compound 1 was determined by an X-ray single-crystal technique and an intramolecular C=O…H-N hydrogen bond and intermolecular C=S…H-N and C=S…H-C hydrogen interactions, which were observed for the crystal structure. The molecular electrostatic potential (MEP) and the Mulliken atomic charges of title compounds 1 and 2 were theoretically calculated and interpreted. Cyclic voltammetric (CV) experiments for the compounds were performed with the glassy carbon electrode. The reduction in potential values of the different functional groups such as nitro and cyano in title compounds were investigated using CV curves.
1. Introduction
N-(aroyl/acyl)-N′-substituted-(aryl/alkyl)-thiourea derivatives are known to exhibit a wide variety of biological activities such as antibacterial and antifungal properties and regulating activities for plant protection in agriculture [1,2,3]. In addition, some of them have been used as ligands in the extraction, separation, and determination of heavy metals such as Fe and Zn ions [4,5]. These types of ligands have been used as a chemosensor for anions such as F−, CN−, OAc−, etc., and as a potentiometric sensor for heavy metals in analytical applications [6,7]. Aroylthiourea derivatives with different substituents have been successfully employed in environmental control as ion-selective electrodes [8,9]. Furthermore, the synthesis of biologically important heterocyclic scaffolds, such as thiohydantoins has also been used some arylthiourea derivatives [10]. Additionally, some aroylthioureas were used in the synthesis of benzoylaminoimidazolone and aminoimidazolone derivatives [11], bicyclic 2-aminothiazolyl compounds [12] and some tetrazoles and guanidines [13].
In this study, two thiourea compounds named N-benzoyl-N′-(4′-cyanophenyl)thiourea (1) and N-(4-nitrobenzoyl)-N′-(4′-cyanophenyl)thiourea (2) were synthesized (Scheme 1), and their structures were characterized by elemental analysis, FT-IR and 1H-NMR, and 13C-NMR spectroscopic techniques.
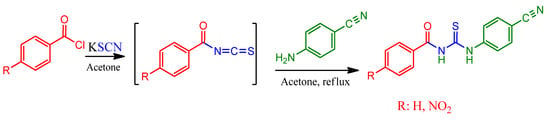
Scheme 1.
The synthesis of N-Benzoyl/(4-nitrobenzoyl)-N′-(4′-cyanophenyl)thioureas.
Since N-benzoyl-N′-(4′-cyanophenyl)thiourea (1) forms a single-crystal structure; the crystal structure was clarified by the X-ray single crystal technique. The intramolecular hydrogen bond and intermolecular hydrogen interactions in the crystal structure for the title compound 1 were interpreted.
The molecular structure of title compounds 1 and 2 were optimized using DFT in the ground state by B3LYP [14] method with the 6-311G(d,p) basis sets included in Gaussian 09 program [15]. Molecular electrostatic potential (MEP) and the Mulliken atomic charges using (DFT) with B3LYP/6-311G(d,p) level of title compounds 1 and 2 were theoretically calculated.
These compounds include functional groups such as nitro and cyano groups, as well as carbonyl and thiocarbonyl functional groups that shown tautomeric equation [16]. To know the redox potential values of these functional groups in their structures, electrochemical potentials of the title compounds as CV curves were examined.
2. Results
2.1. Spectroscopic Studies
The title compounds have been synthesized and characterized by elemental analysis, FT-IR as well as 1H- and 13C-NMR spectroscopic methods. The molecular structure of compound 1 due to the obtained crystals was determined by a single-crystal X-ray diffraction study. All expected frequencies of the ν (N-H), ν(C≡N), ν(C=O), ν(C=N) and ν(C=S) were observed in the infrared spectra of the title compounds. The FT-IR data show the important stretching bands for ν(N-H) and ν(C=O) at 3229 and 1662 cm−1 for compound 1 and 3212 and 1675 cm−1 for the compound 2, respectively, (Figures S1 and S2) [17]. The lower force constant of the (C=O) stretching modes found for the title compounds is related to the conjugated resonance with the phenyl ring as well as the intra-molecular C=O…H—N hydrogen bond, compared with the ordinary carbonyl absorption (~1710 cm−1). The stretching frequencies observed at 2223 and 2225 cm−1 for the title compounds confirm that the C≡N functional group is located on the other side of the aromatic ring, respectively [18]. In addition, strong bands observed at 1518 and 1514 cm−1 for the title compounds, respectively, highlight the presence of the stretching vibration of thiouredio moiety (νN-CS-N). A strong band at 1262.9 and 1262.5 cm−1 are assigned as stretching vibration bands of the thiocarbonyl group (C=S) for the title compounds, respectively [19]. The medium intensity IR absorption bands observed 834 and 854 cm−1 for the title compounds are assigned to the ν(C=S) mode of the thiourea derivatives. The formation of C=S…H- intermolecular hydrogen bonds seem to strongly affect the frequency of the (C=S) mode [20,21]
The 1H-NMR spectrum of the title compounds indicate that the synthesized structures are aroyl thioureas derivatives. The signals that appeared at δH 12.70, 11.69 ppm and 12.47 and 12.04 ppm are characteristic of the N-H protons of the thiourea moiety for the title compounds 1 and 2, respectively [22]. There are multiple signals at δH 7.50–8.30 ppm which corresponds to the aromatic protons on phenyl rings in 1 and 2. The chemical shifts of the hydrogen atoms on the benzene and cyano-benzene rings are in a lower field than that of the nitro-benzene ring (Figures S3 and S4).
The most de-shielded 13C-NMR signals correspond to C=S and C=O groups. The carbon atoms of the thiocarbonyl group (C=S) at δC 179.8 ppm and 179.4 ppm for the compounds 1 and 2 were showed the highest values, whereas the signals of carbonyl groups (C=O) at 168.6 and 167.0 ppm also appeared more de-shielded in the NMR spectra, respectively. The carbon signal of the nitrile group generally appears at δC 110–120 ppm, downfield from TMS [23]. From the 13C-NMR spectrums, the characteristic -C≡N signals appeared at δC 119.2 and 119.1 ppm for the compounds 1 and 2, respectively. Moreover, the signals of aromatic carbon atoms for these compounds 1 and 2 were observed in the range of 108.6–142.7 and 108.7–150.4 ppm, respectively. The 1H-NMR and 13C-NMR data are shown in Figure 1, Figures S5 and S6. Compound 1 is among the previously synthesized compounds, and its spectroscopic data in the FT-IR, 1HNMR, 13C-NMR spectra generally agree [11].
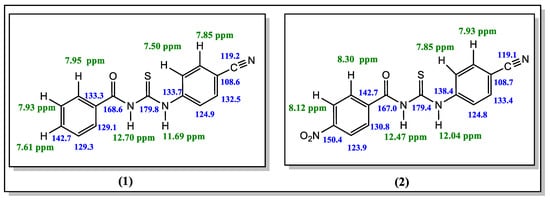
Figure 1.
1H and 13C-NMR chemical shift of the title compounds 1 and 2 in DMSO-d6.
2.2. Single-Crystal X-ray Studies of Title Compound 1
The X-ray result of the synthesized thiourea derivative 1 at room temperature exhibits that the compound (C15H11N3OS) crystallized in triclinic with the space group P-1, a = 4.0684 (3) Å, b = 12.3410 (11) Å, c = 14.7486 (13) Å, α = 69.009 (3)⁰, β = 89.918 (3)⁰, γ = 83.018 (3)⁰, V = 685.52 (10) Å3, Z = 2, T= 296 K, δ = 1.363 Mg.m−3, F000 = 292, µ = 0.23 mm−1, 3430 independent reflections, −5 ≤ h ≤ 5, −16 ≤ k ≤ 16, −19 ≤ l ≤ 19, 2170 reflections with I > 2σ(I) Rint = 0.069. A weighting scheme of the form w = 1/[σ2(Fo2) + (0.1242P)2 + 0.0055P] was introduced, where P = (Fo2 + 2Fc2)/3. The final R1 was 0.064 [F2 > 2σ(F2)] and wR2(F2) was 0.213, Δρmax = 0.61 e Å−3, Δρmin = −0.38 e Å−3., respectively. Crystal data and structure refinement parameters for the title compound 1 are listed in Table S1. The molecular structure of the title compound 1 is shown in Figure 2. Bond lengths, bond angles and torsion angles are presented in Table S2. The C7—O1 and C8—S1 bonds show a typical double bond character with bond lengths of 1.220 (3) Å and 1.650 (3) Å, respectively. The C=O bond length is 1.220 (3) Å, longer than the average C=O bond length (1.200 Å), which is due to intramolecular hydrogen bonding (N2—H2A…O1: 2.646 (3) Å). All the C—N bonds lengths, C7—N1 1.380 (3) Å, C8—N1 1.400 (3) Å, C8—N2 1.342 (3) Å, C9—N2 1.414 (3) Å, also indicate a partial double bond character. The C7—N1 bond is adjacent to the carbonyl group and thus slightly shorter than the C8-N2 bond. In the title compound 1, the C≡N triple bond length is 1.140 (4) Å. The cyano group lies in the plane of the phenyl ring which the maximum deviation from the mean plane belongs to the C9 atom with −0.008 Å.
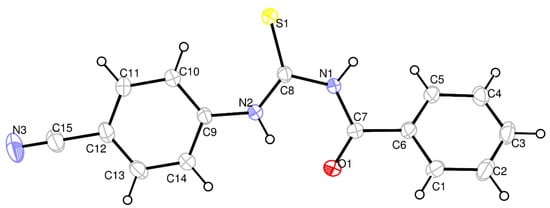
Figure 2.
The ORTEP drawing of the N-Benzoyl-N′-(4′-cyanophenyl)-thiourea (1) with thermal ellipsoid at 30% level.
The molecular packing involves a classical intramolecular hydrogen bonding, N2—H2 …O1, and two types of weak intermolecular interactions between N-H…S and C-H…S atoms are shown in Figure 3 and Table S3. The N-H…O type intramolecular hydrogen bond generates an S(6) ring motif [24]. The molecules are dimerized due to the N1—H1…S1, and C5—H5…S1 types of weak intermolecular interactions bonding with R2 2(8) and R2 2(14) ring motifs.
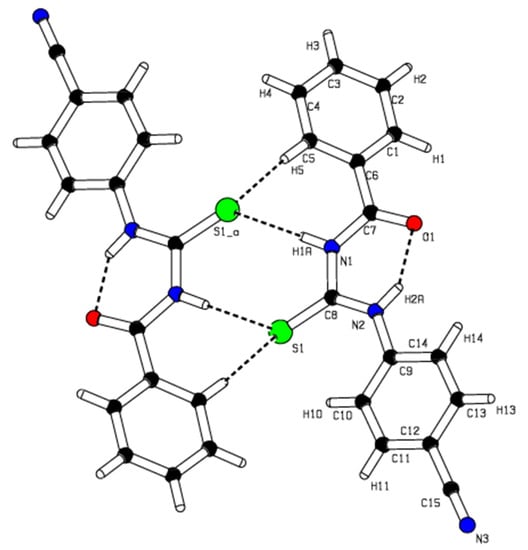
Figure 3.
A view of the intermolecular hydrogen bonds N-H…S, and C-H…S for compound I.
2.3. Molecular Electrostatic Potential (MEP) and Mulliken Atomic Charges for Compounds (1 and 2)
The MEP is a colored plot mapped onto isosurfaces of electron density. It demonstrates the probable positions for electrophilic attacks or nucleophilic reactions and is useful in processes of biological comprehension and interactions of hydrogen bonding [25]. The MEP mapped surface of the compounds 1 and 2 were calculated by DFT/B3LYP at 6-311G (d,p) basis set and MEP surface are plotted in Figure 4. Red, blue, and green colors represent regions of the most electronegative, most electropositive electrostatic and zero potentials, respectively [26]. The color code of these maps is in the range between −5.996 a.u. (deepest red) and 5.996 a.u. (deepest blue) in compound 1, and −6.894 a.u. (deepest red) and 6.894 a.u. (deepest blue) in compound 2. As can be seen from the MEP map of the title compounds 1 and 2, the MEP maps show that regions with negative potential are mainly localized over the nitrogen atom of the cyano group, as well as electronegative oxygen and sulfur atoms. The maps also show that the positive potential regions are more above the hydrogen atoms (N1-H1A, N2-H2A) of the urea moiety and around the H atoms of the phenyl rings, and especially C5-H5. On the other hand, it is observed that the presence of the nitro group partially changed the negative and positive potentials. In the presence of the nitro group, it is observed that the electron density in the cyano and thiocarbonyl groups decreases as well as the electron density changes in the nitrogens in the urea (N1 and N2) for compound 2.
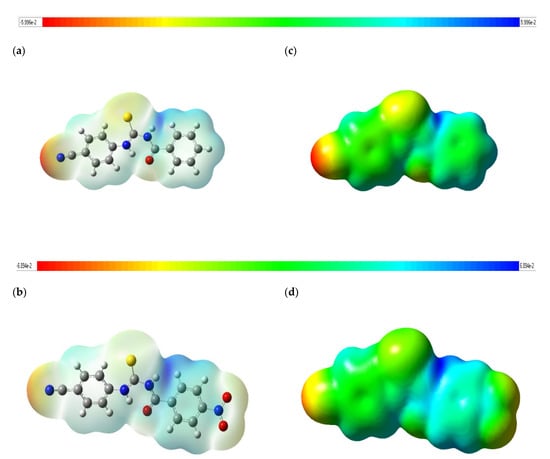
Figure 4.
Molecular electrostatic potential map calculated at B3LYP/6-311G(d,p) level for the title compounds 1 (a,c) and 2 (b,d).
The Mulliken atomic charges have a significant role in the determination the electron population for each atom of the molecular system. Figure 5 exhibits the calculated Mulliken atomic charges for atoms by DFT/B3LYP at 6-311G(d,p) basis set and the graphical representation of Mulliken atomic charge distributation in the title compounds 1 and 2. The presence of the nitro group at the para position affected the electron densities (Table S4).
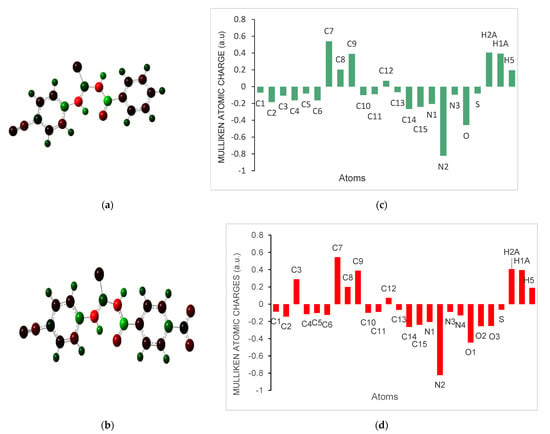
Figure 5.
(a,b) The Mulliken charges (in a.u.) of the C, O, N, S, and some H atoms for the title compounds 1 and 2 were calculated by DFT B3LYP/6-311G (d,p) basis set; (c,d) The graphical representation of Mulliken atomic charge distributation in the title compounds 1 and 2, respectively.
2.4. Electrochemical Studies
The electrochemical properties of aroylthiourea derivatives were investigated further by using cyclic voltammetry analysis to determine the redox potentials. The cyclic voltammetry (CV) curves of the compounds are given in Figure 6. The curves of compound 1 4.0 × 10−3 M and (II) 4.0 × 10−3 M were recorded in acetonitrile (ACN) solution containing 0.1 M lithium perchlorate (LiClO4) as a supporting electrolyte at a scan rate of 0.01 V.s–1 in the potential range of 0.0 V and −2.0 V on glassy carbon electrode (GCE). Ag/AgCl electrode and platinum wire was used as reference and counter electrodes, respectively, in the three electrodes cell.
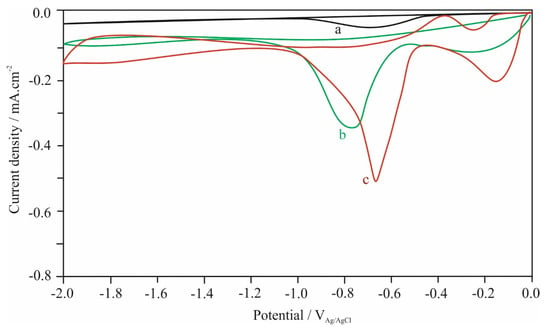
Figure 6.
Cyclic voltammograms obtained in 0.1 M LiClO4 acetonitrile solution at a scan rate of 0.01 V/s for (a) only, (b) 0.004 M compound 1, (c) 0.004 M compound 2.
One cathodic peak was observed for 1 whereas two cathodic peaks were observed for compound 2. As could be seen from Figure 6, the first peak observed about –0.130 mV for 2 can be attributed to the reduction in the -NO2 (nitro) group in its structure [27]. The second peaks observed as approximately –0.770 mV (b) for 1 and –0.650 mV (c) for 2 were thought to result from the reduction in C≡N groups of the molecules. It is seen that; the presence of the -NO2 group affects the reduction potential of the cyano group and shifts it to more positive values. Additionally, it was observed that the C=O and C=S functional groups were not reduced due to the tautomeric equilibrium in acetonitrile solution [16].
3. Materials and Methods
3.1. General Information
All standard chemicals and solvents were sourced from Merck (Darmstadt, Germany) and used without further purification. Melting points were measured on an Electro Thermal IA 9100 apparatus (Cole-Palmer, Staffordshire, UK) using a capillary tube. The Infrared absorption spectrums were measured on a Perkin Elmer BX II (Shelton, CT, USA) spectrophotometer from 4000 to 650 cm−1. The 1H- and 13C-NMR spectra were recorded in DMSO-d6 solution on a Bruker AVANCE DPX NMR spectrometer operating at 400 and 101.6 MHz (Bruker GmbH, Mannheim, Germany) spectrometer with chemical shifts relative to tetramethyl silane and chemical shifts are reported in parts per million (δ/ppm). Elemental analyses were performed on a LECO CHNS-932 Elemental Analyzer (Saint Joseph, MI, USA). For the X-ray analysis, Data collection: Bruker D8 QUEST diffractometer, Bruker SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick 2008); program(s) used to refine structure: SHELXL2014 (Sheldrick 2014); molecular graphics: Bruker SHELXTL; software used to prepare material for publication: Bruker SHELXTL [28,29]. The electrochemical measurements were carried out by using electrochemical analyzer (Gamry, Interface 1000).
3.2. The Synthesis of N-Benzoyl-N′-(4′-Cyanophenyl)Thiourea (1) and N-(4-Nitrobenzoyl)-N′-(4′-Cyanophenyl)Thiourea (2)
A solution of benzoyl chloride (1.40 g, 10 mmol) or 4-nitrobenzoyl chloride (1.85 g, 10 mmol) and potassium thiocyanate (0.969 g, 10 mmol) in acetone (20 mL) was refluxed with stirring for 30 min. After then, the solution of 4-cyanoaniline (1.18 g, 10 mmol) in acetone (20 mL) was added dropwise to the aroyl isothiocyanates for ca. 15 min at ambient temperature. When the solution was added completely, the resulting solution was refluxed for 2.5 and 3.0 h for compound 1 and 2, by controlling the progress of the reaction with TLC, respectively. After cooling, the solution was poured into a beaker containing an ice-water mixture. The white precipitate for compound 1 and the yellowish precipitate for compound 2 were filtered off and washed with distilled water several times and then dried under vacuum. Compound 1 was recrystallized from tetrahydrofuran: ethyl acetate (1:1).
3.2.1. N-Benzoyl-N′-(4′-Cyanophenyl)Thiourea, C15H11N3OS, (1)
Yield 87% of the slightly pale-yellow crystals; m.p.: 168–169 ℃; Elemental analysis: Anal. calcd: C, 64.04; H, 3.94; N, 14.94; S, 11.40%; found: C, 63.96; H, 3.98; N, 14.82; S, 11.60%. FT-IR [ATR (solid), ν.cm−1]: 3229 (N-H, stretch); 3030 (C-Harom); 2223 (C≡N); 1662 (C=O); 1592 (N-H, bending); 1518 (thiocarbonyl, CS-N); 1262 (N-C=S, thioureido); 1162 (carbonyl, CO-N); 834 (C=S). 1H-NMR (DMSO-d6): δ = 12.70 (s, 1H, C=ONHC=S); 11.69 (s, 1H, C=SNHC=Car); 7.95 (d, J = 8.49 Hz, 2H, CHph), 7.93 (d, J = 7.79 Hz, 2H, CHph), 7.85 (d, J = 19.1 Hz, CHar), 7.61 (t, J = 7.41 Hz, 1H, CHph),, 7.50 (d, J = 7.73 Hz, 2H, CHar); 13C-NMR (DMSO-d6): 179.8 (C=S); 168.6 (C=O); 142.7, 133.7 133.3 132.5 129.3, 129.1, 124.9, 108.6 (ArC); 119.2 (C≡N).
3.2.2. N-(4-Nitrobenzoyl)-N´-(4′-Cyanophenyl)Thiourea, C15H10N4O3S, (2)
Yield 84% of the pale-yellow crystal; m.p.: 206–207 ℃; Elemental analysis: Anal. calcd: C, 55.21; H, 3.09; N, 17.17; S, 9.83%; found: C, 55.13; H, 3.05; N, 17.10; S, 10.02%. FT-IR [ATR (solid), ν.cm−1]: 3212 (N-H); 3070, 3005 (C-Harom); 2225 (C≡N); 1675 (C=O); 1582 (N-H, bending); 1514 (thiocarbonyl, CS-N); 1335 (N-C=S, thioureido); 1156 (carbonyl, CO-N); 854 (C=S). 1H-NMR (DMSO-d6): δ = 12.47 (s, 1H, C=ONHC=S); 12.04 (s, 1H, C=SNHC=Car.); 8.30 (d, J = 8.82 Hz, 2H, CHar); 8.12 (d, J = 8.71 Hz, 2H, CHar), 7.93 (d, J = 8.68 Hz, 2H, CHar), 7.85 (d, J = 10.63 Hz, 2H, CHar); 13C-NMR (DMSO-d6): 179.4 (C=S); 167.0 (C=O); 150.4, 142.7, 138.4, 133.4, 130.8, 124.8, 123.9, 108.7 (ArC); 119.1 (C≡N).
4. Conclusions
Two thioureas, N-benzoyl-N′-(4′-cyanophenyl)thiourea (1) and N-(4-nitrobenzoyl)-N′-(4′-cyanophenyl)thiourea (2) were prepared in high yield via aroylisothiocyanate salts. After the structure of the title compounds were characterized via the FT-IR and 1H-, 13C-NMR spectroscopic methods. Cyclic voltammetry has been used to investigate the reduction potential of the different functional group in the molecules. It has been observed that the nitro group is more easily reduced than the cyano group due to different conjugation. The 3D structural determination of 1 was established by X-ray crystallography and confirmed the structure in the solid state as anticipated on spectroscopic data. The molecular conformation is stable by an intramolecular N-H…O hydrogen bond and intermolecular N-H…S and C-H…S hydrogen interactions. The charge distribution in title compounds were theoretically examined as MEP mapping and Mulliken atomic charges. It has been observed that the presence of the nitro group in the para position of phenyl ring causes the changes in the spectroscopic and electrochemical data, as well as the theoretical calculations of the structures with the same functional group.
Supplementary Materials
The following supporting information can be downloaded. Figure S1: IR-spectrum of 1; Figure S2: IR-spectrum of 2; Figure S3: 1H-NMR of 1; Figure S4: 1H-NMR of 2; Figure S5: 13C-NMR of 1; Figure S6: 13C-NMR of 2; Tables S1–S3: Crystal data of 1; Table S4: Mulliken charges of 1 and 2. Crystallographic data for 1 reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with quotation number CCDC-2064593, and it can be obtained free of charge on application to CCDC 12 Union Road, Cambridge CB2 1EZ, UK [Fax: (internet.) + 44(1223)336–033, E-mail: deposit@ ccdc.cam.ac.uk].
Author Contributions
F.A.: designed the study, carried out the synthesis and electrochemical study, characterized the structures with spectroscopic methods, analyzed the data and wrote the paper; N.B.A.: carried out the X-ray analysis and solved the structure, carried out the theoretical calculations. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Çanakkale Onsekiz Mart University Grants Commission for a research grant (Project Number: FYL-2018-2583).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahdavi, M.; Shirazi, M.S.; Taherhani, R.; Saeedi, M.; Alipour, E.; Moghadam, F.H.; Moradi, A.; Nadri, H.; Emami, S.; Firoozpour, L.; et al. Synthesis, biological evaluation and docking study of 3-aroyl-1-(4-sulfamoylphenyl)thiourea derivatives as 15-lipoxygenase inhibitors. Eur. J. Med. Chem. 2014, 82, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Flörke, U.; Erben, M.F. A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J. Sulfur Chem. 2014, 35, 318–355. [Google Scholar] [CrossRef]
- Koch, K.R. New chemistry with old ligands: N-alkyl- and N,N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord. Chem. Rev. 2001, 216–217, 473–488. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Lusta, Z.I.; Zubi, A.E. Synthesis and use of thiourea derivative (1-phenyl-3-benzoyl-2-thiourea) for extraction of cadmium ion. Int. J. Chem. Mater. Sci. Eng. 2014, 8, 118–120. [Google Scholar]
- Aydin, F.; Tunoǧlu, N.; Aykaç, D.; Arslan, N.B.; Kazak, C. Synthesis and structural X-ray analysis of 1,1′-(naphthalene-1,8-diyl)-3,3′-dibenzoyl-bisthiourea and its use as anion-binding receptor. Turkish J. Chem. 2012, 36, 764–777. [Google Scholar] [CrossRef]
- Aydin, F.; Dagci, E. N,N′-Bis-(4-nitrophenylcarbamothioyl) phthalamide. Molbank 2013, 2013, M809. [Google Scholar] [CrossRef]
- Saad, F.A.; Knight, J.C.; Kariuki, B.M.; Amoroso, A.J. Co-ordination behaviour of a novel tristhiourea tripodal ligand; Structural variations in a series of transition metal complexes. Dalt. Trans. 2016, 45, 10280–10288. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Marín, L.; Estévez-Hernández, O.; Rojas-Lima, S.; Alonso-Chamarro, J. Aroylthioureas: New organic ionophores for heavy-metal ion selective electrodes. J. Chem. Soc. Perkin Trans. 2001, 1, 2211–2218. [Google Scholar] [CrossRef]
- Erşen, D.; Ülger, M.; Mangelinckx, S.; Gemili, M.; Şahin, E.; Nural, Y. Synthesis and anti(myco)bacterial activity of novel 5,5-diphenylpyrrolidine N-aroylthiourea derivatives and a functionalized hexahydro-1H-pyrrolo[1,2-c]imidazole. Med. Chem. Res. 2017, 26, 2152–2160. [Google Scholar] [CrossRef]
- Dražić, I.T.; Vazdar, K.; Vazdar, M.; Đaković, M.; Mikecin, A.-M.; Kralj, M.; Malnar, M.; Hećimović, S. Synthesis of new 2-aminoimidazolones with antiproliferative activity via base promoted amino-beta-lactam rearrangement. Tetrahedron 2015, 71, 9202–9215. [Google Scholar] [CrossRef]
- Rong, W.; Shuang, H.; Xiaojing, D.; Daijie, C.; Lei, S.; Liujia, Q.; Zhong, L.; Xiaoyong, X. Synergism of fused bicyclic 2-aminothiazolyl compounds with polymyxin B against Klebsiella pneumoniae. Medchemcomm 2017, 8, 2060–2066. [Google Scholar] [CrossRef]
- Bajivali, S.; Mohan, S.; Ramana, T.; Rao, K.P. Iodine-Mediated Multi-Component Reactions: Readily Access to Tetrazoles and Guanidines. Lett. Org. Chem. 2021, 18, 382–388. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Aydın, F.; Ünver, H.; Aykaç, D.; Iskeleli, N.O. Spectroscopic studies and structure of 4-(3-benzoylthioureido)benzoic acid. J. Chem. Crystallogr. 2010, 40, 1082–1086. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, W.; Zhang, Z. Structural and spectroscopic study on N-2-fluorobenzoyl-N′-4-methoxyphenylthiourea. J. Mol. Struct. 2007, 828, 46–53. [Google Scholar] [CrossRef]
- Kumar, V.; Panikar, Y.; Palafox, M.A.; Vats, J.K.; Kostova, I.; Lang, K.; Rastogi, V.K. Ab-initio calculations, FT-IR and FT-Raman spectra of 2-chloro-6-methyl benzonitrile. Indian J. Pure Appl. Phys. 2010, 48, 85–94. [Google Scholar]
- Rao, C.N.R.; Venkataraghavan, R. The C=S stretching frequency and the “-N-C=S bands” in the infrared. Spectrochim. Acta 1962, 18, 541–547. [Google Scholar] [CrossRef]
- Lenthall, J.T.; Foster, J.A.; Anderson, K.M.; Probert, M.R.; Howard, J.A.K.; Steed, J.W. Hydrogen bonding interactions with the thiocarbonyl π-system. Cryst. Eng. Comm. 2011, 13, 3202–3212. [Google Scholar] [CrossRef]
- Gumus, I.; Solmaz, U.; Binzet, G.; Keskin, E.; Arslan, B.; Arslan, H. Supramolecular self-assembly of new thiourea derivatives directed by intermolecular hydrogen bonds and weak interactions: Crystal structures and Hirshfeld surface analysis. Res. Chem. Intermed. 2019, 45, 169–198. [Google Scholar] [CrossRef]
- Osborne, A.G. Long range cyano 13C-1H coupling constants in some cyanopyridines and benzonitriles. Spectrochim. Acta Part A 1997, 53, 2475–2480. [Google Scholar] [CrossRef]
- Löser, R.; Pitzschler, R.; Köckerling, M. Synthesis and X-ray crystal structure of N′-Cyano-N,N′-dimethyl-4-nitrobenzohydrazide. Crystals 2017, 7, 290. [Google Scholar] [CrossRef]
- Aydin, F.; Aykaç, D.; Burcu Arslan, N.; Kazak, C. Synthesis, characterization, and crystal structure of bis[4-(3′-benzoyl)thiocarbamidophenyl]ether. Crystallogr. Rep. 2014, 59, 955–960. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Luque, F.J.; Orozco, M.; Bhadane, P.K.; Gadre, S.R. SCRF calculation of the effect of water on the topology of the molecular electrostatic potential. J. Phys. Chem. 1993, 97, 9380–9384. [Google Scholar] [CrossRef]
- Olson, E.J.; Isley, W.C.; Brennan, J.E.; Cramer, C.J.; Bühlmann, P. Electrochemical Reduction of 2,4-Dinitrotoluene in Aprotic and pH-Buffered Media. J. Phys. Chem. C 2015, 119, 13088–13097. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97: Program for the Solution Crystal Structure; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G. SHELXL-97: Program for the Refinement Crystal Structure; University of Gottingen: Göttingen, Germany, 1997. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).