Abstract
In the present work, construction of double-charged cationic amphiphilic 1,1′-{[3,5-bis(dodecyl¬oxy-carbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) dibromide (7) was performed in four steps. Dodecyl 3-oxobutanoate (1) was condensed with thiophene-3-carbaldehyde (2) which was necessary for Hantzsch cyclisation dodecyl (E/Z)-3-oxo-2-(thiophen-3-ylmethylene)butanoate (3). Two-component Hantzsch type cyclisation of dodecyl (E/Z)-3-aminobut-2-enoate (4) and dodecyl (E/Z)-3-oxo-2-(thiophen-3-ylmethylene)butanoate (3) gave 3,5-bis(dodecyloxycarbonyl)-2,6-dimethyl-4-(thiophen-3-yl)-1,4-dihydropyridine (5). Bromination of compound 5 followed by nucleophilic substitution of bromine with pyridine gave the desired cationic amphiphilic 1,4-dihydropyridine 7. The obtained target compound 7 and new intermediates 3, 5 and 6 were fully characterised by IR, UV, 1H NMR, 13C NMR, HRMS or microanalysis. Characterisation of nanoparticles formed by the cationic 1,4-dihydropyridine 7 in an aqueous solution was performed by DLS measurements.
1. Introduction
1,4-Dihydropyridines (1,4-DHP) belong to a class of six-membered nitrogen-containing heterocycles [1]. Among the family of dihydropyridines, most notable is Hantzsch type 1,4-DHP, which is regarded as a crucial scaffold that exists across a range of calcium channel antagonists that are widely used for the treatment of hypertension, angina pectoris, cardiac arrhythmias and other disorders [2,3,4]. Functionalisation of the 1,4-DHPs is a powerful tool for the construction of pharmacologically and biologically important compounds [5,6]. Thus, polyfunctional pyridinium amphiphiles on a base of 1,4-DHP were found to be active for DNA delivery. Some representatives of this class were more active than commercially available cationic lipid DOTAP and polymer PEI 25 (25 kDa) for DNA delivery and could be used as transmembrane delivery systems. Amphiphilic 1,4-DHPs also possess self-assembling properties and may form liposomes [7]. The studies of modification of the cationic head-group of the 1,4-DHP molecule showed that the electronic nature of the substituents strongly affected the ability of compounds to bind pDNA and transfer it into the cells [7]. Synthetic polyfunctional lipids based on pyridines as an oxidised form of 1,4-DHPs also exhibit self-assembling properties and may form liposomes. Some representatives of this class show pDNA transfection activity; however, it is lower than for the corresponding 1,4-DHP amphiphiles [8]. Minor modifications, such as elongation by one methylene group of the distance between cationic moieties and the linker, also led to notable changes in the delivery activity of cationic 1,4-DHP [9]. It is possible to fill liposomes formed by amphiphilic 1,4-DHP derivatives with magnetic particles, which show targeted accumulation under the guidance of external magnetic fields [10]. The design of nanoparticle delivery systems possessing biological activities is an attractive strategy for the development of new therapies [11]. The cationic amphiphilic 4-(N-alkylpyridinium)-1,4-DHP representatives with different alkyl chain length and propargyl moiety/ties number, and position exhibited cytotoxic effects on several tumor cell lines and toxicity on Gram-positive and Gram-negative bacteria species and eukaryotic microorganisms besides self-assembling properties [12].
It is known that thiophene-containing derivatives show antimicrobial, analgesic, anti-inflammatory, antihypertensive and antitumor activity in medicine [13]. Recently, it was reported that introduction of bromothiophene moiety in 1,4-DHP scaffold led to the formation of the compounds with antimicrobial and antifungal activities [14]. Because of the great biological potential of these molecules, synthesis of cationic amphiphilic 1,4-DHP 7 with thiophene group was performed.
Hantzsch synthesis remains the most common method for the synthesis of 1,4-DHPs [15,16]. The most common variant of the Hantzsch synthesis consists of the reaction of an aldehyde with an active methylene carbonyl compound and a source of ammonia [17]. Various modifications of this method were also developed and often used when the classic Hantzsch synthesis failed. Frequently, enamines are used instead of active methylene carbonyl compound and a source of ammonia [18]. Aldehydes can be condensed with active methylene compounds leading to α,β-unsaturated ketones. This alternative to a classic Hantzsch synthesis of 1,4-DHPs is also frequently applied [19]. A two-component reaction of α,β-unsaturated ketones with enamines provides a convenient way to obtain 1,4-DHPs and reduced side product formation [20]. Bromination of 2,6-methyl groups of 1,4-DHPs was performed mainly with N-bromosuccinimide (NBS) in methanol [21], but chloroform was also proposed as suitable media for this reaction [22]. The preferred conditions of bromination of 1,4-DHP 2,6-methyl groups are the dropwise addition NBS solution to the reaction mixture [23]. Nucleophilic substitution of bromine with pyridine, substituted pyridines, pyrazine, N-methyl piperidine or N-methyl morpholine has been successfully applied for the synthesis of amphiphilic compounds by our research group [7]. It should be noted that at temperatures above 50 °C 2,6-bis(bromomethyl)-1,4-DHPs may undergo partial or full lactonisation, therefore substitution reactions should be performed at room temperature (r.t.) [22,24].
Herein, we report the synthesis and full characterisation of new 1,1′-{[3,5-bis(dodecyloxycarbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) dibromide (7) through the multistep procedure starting from two-component Hantzsch type cyclisation of dodecyl 3-aminobut-2-enoate (4) and dodecyl 3-oxo-2-(thiophen-3-ylmethylene)butanoate (3). 3-Oxo-2-(thiophen-3-ylmethylene)butanoate (3) was prepared from dodecyl 3-oxobutanoate (1) and thiophene-3-carbaldehyde (2) at r.t. Bromination of 2,6-methyl groups at the 3,5-bis(dodecyloxycarbonyl)-2,6-dimethyl-4-(thiophen-3-yl)-1,4-dihydropyridine (5) followed by nucleophilic substitution of bromine with pyridine gave the desired cationic amphiphilic 1,4-DHP 7. The dynamic light scattering (DLS) measurements of nanoparticles formed by the cationic 1,4-DHP 7 in an aqueous solution were performed.
2. Discussion
The desired target product—1,1′-{[3,5-bis(dodecyloxycarbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) dibromide (7) was obtained using a multistep procedure (Scheme 1). Taking into account the relatively high cost of thiophene-3-carbaldehyde (2) and the fact that the two-component variant of Hantzsch synthesis proceeds with less formation of side products, the coupling of aldehyde with active methylene compounds should be performed prior to the cyclisation. The first stage was preparation of dodecyl (E/Z)-3-oxo-2-(thiophen-3-ylmethylene)butanoate (3) from dodecyl 3-oxobutanoate [25] (1) and thiophene-3-carbaldehyde (2) in isopropanol at r.t. in the presence of three drops of acetic acid, and three drops of piperidine with stirring of reaction mixture for 4 days at r.t. Product 3 was obtained in 94% yield as a mixture of E/Z isomers in ratio 1:2 according to 1H NMR data. The next step was two-component Hantzsch type cyclisation of dodecyl 3-aminobut-2-enoate [21] (4) and dodecyl (E/Z)-oxo-2-(thiophen-3-ylmethylene)butanoate (3) according to previously described methodology in the presence of 10 mol% of 1-butyl-3-methylimidazolium chloride as catalyst [21] providing 1,4-DHP 5 in 49% yield. Various catalysts were used in the synthesis of 1,4-DHP derivatives [26]. During the last few decades, ionic liquids as green catalysts were used for different reactions, including Hantzsch reaction. For example, betainium-based ionic liquids were used for the one-pot production of acridinediones through Hantzsch reactions under mild conditions [27], β-cyclodextrin/imidazolium based dicationic ionic liquid in the one-pot, three-component Hantzsch reaction [28], 3-methyl-1-sulfonic acid imidazolium chloride as an acidic ionic liquid in four-component Hantzsch reaction leading to a simple synthetic procedure, short reaction times, less pollution and high yields of the products [29]. Bromination of 2,6-methyl groups of the 3,5-bis(dodecyloxycarbonyl)-2,6-dimethyl-4-(thiophen-3-yl)-1,4-dihydropyridine (5) was performed with NBS according to the previously described procedure [26]. To a solution of 2,6-dimethyl-1,4-DHP 5 in methanol a solution of NBS in methanol was added dropwise, after which the reaction mixture was stirred at r.t. for 3 h. The desired 2,6-bis(bromomethyl)-1,4-DHP 6 was obtained in 68% yield. The last step—nucleophilic substitution of bromine with pyridine—was performed in acetone overnight, giving target 1,1′-{[3,5-bis(dodecyloxycarbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis(pyridin-1-ium) dibromide (7) in 62% yield.
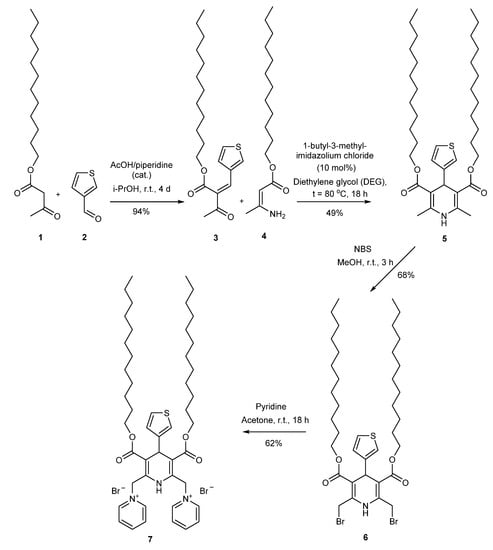
Scheme 1.
Synthesis of 1,1′-{[3,5-bis(dodecyloxycarbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) dibromide (7).
The obtained target compound 7 and new intermediates 3, 5 and 6 were fully characterised by IR, 1H NMR, 13C NMR, UV spectra, HRMS or microanalysis. The characterisation of nanoparticles formed by the cationic 1,4-DHP 7 in an aqueous solution was performed by DLS measurements.
According to 1H NMR, 13C NMR spectra data (see Supplementary Materials) oxo-2-(thiophen-3-ylmethylene)butanoate (3) was obtained as a mixture of E/Z isomers wherein the amount of one isomer is twice that of the other (ratio 1:2). In the IR spectrum, compound 3 demonstrated characteristic peaks at 1766, 1668, 1622 cm−1 characteristic for its C=O and C=C groups. 1H NMR spectrum of didodecyl 2,6-dimethyl-4-(thiophen-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate (5) showed characteristic signals for 1,4-DHP 4-H and NH protons at 5.15 and 5.59 ppm, respectively. In the IR spectrum, compound 5 demonstrated characteristic peaks at 3341 (NH), and peaks at 1699 and 1652 cm−1 of its C=O and C=C groups. Characteristic AB-system signals of 2,6-methylene protons were observed in the 1H NMR spectra of didodecyl 2,6-bis(bromomethyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate (6) and 1,1′-{[3,5-bis(dodecyloxycarbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) dibromide (7) at 4.64/4.88 and 5.87/6.36 ppm, respectively. IR spectrum of compound 6 shows characteristic peaks at 3320 (NH) and peaks at 1700 and 1640 cm−1 of its C=O and C=C groups, while the IR spectrum of compound 7 shows characteristic peaks at 3413 (NH) and peaks at 1689 and 1638 cm−1 of its C=O and C=C groups. UV-Vis spectra of 1,4-DHP derivatives 5–7 showed absorption peaks characteristic for the DHP system at 348–369 nm region.
The self-assembling properties of cationic moieties containing 1,4-DHPs are their characteristic feature. The hydrodynamic average diameters (Z-ave), polydispersity index (PDI), zeta-potential (Z-potential) and stability of nanoparticles formed by 1,4-DHP 7 in aqueous medium were determined by the DLS method. The results are summarised in Table 1. The DLS measurements were performed for a freshly prepared sample (entry 1, Table 1), after storage for 3 days (entry 2, Table 1) and after storage for 1 week (entry 3, Table 1) at r.t.

Table 1.
Values of polydispersity index (PDI), Z-average diameter, zeta-potential of nanoparticles formed by 1,4-DHP amphiphile 7 obtained by DLS measurements. The PDI value describes polydispersity of the sample; the Z-average diameter represents the average hydrodynamic diameter of all nanoparticles in the sample; zeta-potential gives information about the surface charge of nanoparticles.
It was demonstrated that cationic 1,4-DHP 7 formed nanoparticles with the average diameter around 80 nm for the freshly prepared sample, but during the storage for 3 days the average diameter of nanoparticles increased to around 110 and 600 nm after one week. Values of PDI confirmed that during storage, samples became more heterogeneous—the PDI for freshly prepared sample was 0.399, but after storage for 3 days or 1 week the PDI values were 0.589 and 0.627, respectively. Values of the zeta potential of nanoparticles formed by 1,4-DHP 7 indicated the positive surface charge. The obtained zeta potentials over ±20 mV confirmed that the formed nanoparticle solutions were also relatively electrostatically stable [30].
3. Materials and Methods
All reagents were purchased from Acros Organics (Geel, Belgium), Sigma-Aldrich/Merck KGaA (Darmstadt, Germany) or Alfa Aesar (Lancashire, UK) and used without further purification. TLC was performed on silica gel 60 F254 aluminium sheets 20 cm × 20 cm (Merck KGaA, Darmstadt, Germany) or on silica gel 60 RP-18 F254S aluminium sheets 20 cm × 20 cm (Merck KGaA, Darmstadt, Germany). Silica gel of particle size 35–70 µm (Merck KGaA, Darmstadt, Germany) was used for column chromatography. Melting points were recorded on an OptiMelt digital melting point apparatus (Stanford Research Systems, Sunnyvale, CA, USA) and are uncorrected. The 1H and 13C NMR spectra were recorded on a Bruker Avance Neo 400 MHz (Bruker Biospin Gmbh, Rheinstetten, Germany). Chemical shifts of the hydrogen and carbon atoms are presented in parts per million (ppm) and referred to the residual signals of the deuterated CDCl3 (δ: 7.26) solvent for the 1H NMR spectra and CDCl3 (δ: 77.16) solvent for the 13C NMR, respectively. Coupling constants J were reported in hertz (Hz). High-resolution mass spectra (HRMS) were determined on an Acquity UPLC H-Class system (Waters, Milford, MA, USA) connected to a Waters Synapt GII Q-ToF operating in the ESI positive or negative ion mode on a Waters Acquity UPLC® BEH C18 column (1.7 µm, 2.1 × 50 mm, using gradient elution with acetonitrile (0.01% formic acid) in water (0.01% formic acid). Infrared (IR) spectra were recorded with a Prestige-21 FTIR spectrometer (Shimadzu, Kyoto, Japan). UV spectra were recorded on UV-Vis Spectrophotometer (501 UV-Vis CamSpec Spectrophotometer; Spectronic CamSpec Ltd., Leeds, UK). Elemental analyses were determined on an Elemental Combustion System ECS 4010 (Costech International S.p.A., Milano, Italy) at the Laboratory of Chromatography of the Latvian Institute of Organic Synthesis. The DLS measurements of the nanoparticles in an aqueous solution were carried out on a Zetasizer Nano ZSP (Malvern Panalytical Ltd., Malvern, UK) instrument with Malvern Instruments Ltd. Software 7.12.
3.1. Dodecyl (E/Z)-3-oxo-2-(thiophen-3-ylmethylene)butanoate (3)
To a stirred solution of dodecyl 3-oxobutanoate (1) [25] (1.22 g, 4.5 mmol) in isopropanol (30 mL) at r.t. thiophene-3-carbaldehyde (2) (0.39 mL, 4.5 mmol) and three drops of acetic acid, and three drops of piperidine were added, after which the resulting mixture was stirred for 4 d at r.t. The reaction course was monitored by RP-18 silica gel TLC. The reaction mixture was concentrated under reduced pressure and the crude product was purified by C18 RP silica gel column chromatography with MeOH/H2O, (30:70 to 100:0%) to give the desired product 3 (where the ratio between the E/Z isomers is 1:2) as a yellow oil (1.55 g, 94%). Rf = 0.39 (RP-18, MeOH:H2O, 50:50). IR νmax (film) 3099 w, 2928 s, 2854 s, 1733 s, 1668 m, 1622 m. UV-Vis λmax (EtOH): 211 (log ε 4.43), 229 (4.26), 294 (4.70) nm. 1H NMR (400 MHz, CDCl3) δ: 7.66–7.63 (major) and 7.59–7.56 (minor) (m, 1H), 7.62–7.60 (minor) and 7.54–7.52 (major) (m, 1H), 7.35–7.30 (both) (m, 1H), 7.20–7.17 (major) and 7.14–7.11 (minor) (m, 1H), 4.30 (major) and 4.23 (minor) (2xt, 2H, J = 6.7 Hz and J = 6.7 Hz), 2.42 (minor) and 2.39 (major) (2xs, 3H), 1.73–1.65 (both) (m, 2H), 1.35–1.21 (both) (m, 18H), 0.90–0.85 (both) (m, 3H). 13C NMR (101 MHz, CDCl3) δ: 203.6 (minor) and 194.8 (major), 168.3 (major) and 164.9 (minor), 135.1 (major) and 134.9 (minor), 134.5 (major) and 134.0 (minor), 132.9 (major) and 132.16 (minor), 131.15 (major) and 130.7 (minor), 127.8 (minor) and 127.3 (major), 127.10 (major) and 127.04 (minor), 66.2 (major) and 65.8 (minor), 32.1 (both), 31.3 (minor) and 26.7 (major), 29.8; 29.7; 29.6; 29.5; 29.3 (both), 28.7 (minor), 28.5 (major), 26.05 (minor), 26.01 (major), 22.8 (both), 14.3 (both). HRMS of [C21H32O3S + H]+ (m/z) 365.2144; calcd: 365.2150.
3.2. Didodecyl 2,6-dimethyl-4-(thiophen-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate (5)
Dodecyl (E/Z)-3-oxo-2-(thiophen-3-ylmethylene)butanoate (3) (1.55 g, 4.3 mmol) was added to a stirred solution of dodecyl (E/Z)-3-aminobut-2-enoate (4) [21] (1.16 g, 4.3 mmol) in diethylene glycol (20 mL) at r.t., after which 1-butyl-3-methylimidazolium chloride (0.075 g, 0.43 mmol) was added. The resulting mixture was stirred at 80 °C for 18 h. The reaction course was monitored by TLC. After complete conversion the reaction mixture was cooled down to r.t., after which it was diluted with water (50 mL) and ethyl acetate (40 mL). The organic layer was separated, concentrated under reduced pressure and the crude product was purified by flash column chromatography on silica gel with ethyl acetate/petroleum ether (0:100 to 20:80%) to give the product 5 as a colourless powder (1.30 g, 49%) with melting point 69–71 °C (crystallised from EtOH). Rf = 0.32 (Hexane:EtOAc, 90:10). IR νmax (film) 3341 m, 2924 s, 2854 m, 1699 m, 1652 m. UV-Vis λmax (EtOH): 233 (log ε 4.95), 348 (4.49) nm. 1H NMR (400 MHz, CDCl3) δ: 7.13–7.10 (m, 1H), 6.99–6.96 (m, 1H), 6.92–6.90 (m, 1H), 5.59 (s, 1H), 5.15 (s, 1H), 4.13–4.01 (m, 4H), 2.33 (s, 6H), 1.65–1.57 (m, 4H), 1.33–1.23 (m, 36H), 0.90–0.85 (m, 6H). 13C NMR (101 MHz, CDCl3) δ: 167.7, 147.9, 144.3, 127.7, 124.7, 120.4, 103.8, 64.1, 34.7, 32.1, 29.8, 29.83, 29.78, 29.7, 29.5, 29.4, 28.9, 26.2, 22.8, 19.7, 14.3. HRMS of [C37H61NO4S + H]+ (m/z) 616.4398; calcd: 616.4400.
3.3. Didodecyl 2,6-bis(bromomethyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate (6)
To a stirred solution of didodecyl 2,6-dimethyl-4-(thiophen-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate (5) (0.30 g, 0.49 mmol) in methanol (40 mL) at r.t. N-bromosuccinimide (NBS) (0.18 g, 0.98 mmol) in methanol (10 mL) was added dropwise and resulting mixture was stirred for 3 h at r.t. The reaction was monitored by TLC. The reaction mixture was concentrated under reduced pressure and the crude product was purified by flash column chromatography on silica gel with ethyl acetate/petroleum ether (0:100 to 15:85%) to give the product 6 as a yellow powder (0.26 g, 68%) with melting point 76–78 °C (MeOH). Rf = 0.62 (Hexane:EtOAc, 90:10). IR νmax (film) 3320 w, 3110 w, 2924 s, 2853 m, 1700 m, 1640 m, 1506 m. UV-Vis λmax (crystallised from EtOH): 221 (log ε 4.85), 255 (4.74), 369 (4.36) nm. 1H NMR (400 MHz, CDCl3) δ: 7.17–7.14 (m, 1H), 7.00–6.96 (m, 2H), 6.48 (s, 1H), 5.19 (s, 1H), 4.88 and 4.64 (AB-system, 2H and 2H, J = 11.5 Hz), 4.19–4.06 (m, 4H), 1.68–1.59 (m, 4H), 1.35–1.21 (m, 36H), 0.91–0.85 (m, 6H). 13C NMR (101 MHz, CDCl3) δ: 166.3, 145.5, 142.0, 127.3, 125.4, 121.4, 105.7, 65.0, 35.3, 32.1, 29.83, 29.80, 29.77, 29.7, 29.5, 29.4, 28.8, 27.4, 26.2, 22.8, 14.3. Anal. calc. for C37H59Br2NO4S: C, 57.44; H, 7.69; N, 1.81; S, 4.14 found: C, 57.44; H, 8.04; N, 1.51; S, 4.00.
3.4. 1,1′-((3,5-Bis{[dodecyloxycarbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis(pyridin-1-ium) dibromide (7)
To a stirred solution of didodecyl 2,6-bis(bromomethyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate (6) (0.20 g, 0.26 mmol) in acetone (5 mL) at r.t. pyridine (0.042 mL, 0.52 mmol) was added and the resulting mixture was stirred at r.t. for 18 h. The reaction was monitored by RP-18 silica gel TLC. The precipitate was filtered off and dried under vacuum to give the product 7 as a light-yellow powder (0.15 g, 62%) with melting point 161 °C with decomposition (crystallised from acetone). Rf = 0.18 (RP-18, MeOH:CHCl3:AcOH, 49:49:2). IR νmax (KBr) 3413 w, 3056 w, 2923 s, 2852 m, 1689 m, 1638 s, 1517 m. UV-Vis λmax (EtOH): 221 (log ε 4.90), 249 (4.71), 357 (4.21), 437 (3.70) nm. 1H NMR (400 MHz, CDCl3) δ: 10.83 (s, 1H), 9.27 (d, 4H, J = 6.1 Hz), 8.67 (t, 2H, J = 7.7 Hz), 8.23–8.15 (m, 4H), 7.24–7.20 (m, 1H), 7.09–7.06 (m, 1H), 7.00–6.96 (m, 1H), 6.36 and 5.87 (AB-system, 2H and 2H, J = 13.8 Hz), 5.24 (s, 1H), 4.17–4.05 (m, 4H), 1.67–1.57 (m, 4H), 1.34–1.20 (m, 36H), 0.91–0.85 (m, 6H). 13C NMR (101 MHz, CDCl3) δ:.166.4, 147.2, 145.3, 144.8, 138.4, 129.1, 127.1, 126.2, 122.3, 109.7, 65.6, 57.5, 32.0, 29.8, 29.76, 29.7, 29.5, 29.4, 28.6, 26.1, 22.8, 14.2. Anal. calc. for C47H69Br2N3O4S with 5.5% of H2O: C, 57.25; H, 7.67; N, 4.26; S, 3.25 found: 56.95; H, 7.34; N, 3.99; S, 2.90.
3.5. Self-Assembling Properties of Compound 7 by Dynamic Light Scattering Measurements
The sample for the DLS studies was prepared by dispersing compound 7 in an aqueous solution at a concentration 0.5 mM by sonication using a bath type sonicator (Cole Parmer Ultrasonic Cleaner 8891CPX (USA)). The sample was sonicated for 30 min at 60 °C. Liposomes dispersion in water were homogenised using 15 passes in a hand-held, small-scale extruder (Avestin Europe GmbH, Mannheim, Germany) with polycarbonate nucleopore filters (100 nm, Whatman, Frisenette, Knebel, Denmark). The DLS measurements of the nanoparticles in an aqueous solution were carried out on a Zetasizer Nano ZSP (Malvern Panalytical Ltd., Malvern, UK) instrument with Malvern Instruments Ltd. Software 7.12, using the following specifications—medium: water; refractive index: 1.33; viscosity: 0.8872 cP; temperature: 25 °C; dielectric constant: 78.5; nanoparticles: liposomes; refractive index of materials: 1.60; detection angle: 173°; wavelength: 633 nm. Data were analysed using the multimodal number distribution software that was included with the instrument. The measurements were performed in triplicate in order to check their reproducibility.
Supplementary Materials
1H NMR, 13C NMR, UV-Vis and HRMS spectra of compounds 3, 5–7.
Author Contributions
Conceptualisation was conducted by K.P. and R.R.; methodology and experimental works were conducted by M.R., N.P. and A.P.; data analysis, writing and editing of the paper were conducted by A.P., M.R., S.V. and A.S.; project administration and supervision was conducted by K.P. and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Joint Ukraine-Latvia R&D Project “Design and study of physicochemical properties and bioactivity of self-assembling calixarene and dihydropyridine hybrids for DNA delivery’’, grant number LV-UA/2020/3.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We are indebted to Marina Petrova for recording the NMR spectra, to Solveiga Grinberga for the mass spectral analyses and Emma Sarule for the elemental analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- & 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef] [Green Version]
- Triggle, D.J. The 1,4-dihydropyridine nucleus: A pharmacophoric template part 1. Actions at ion channels. Mini-Rev. Med. Chem. 2003, 3, 215–223. [Google Scholar] [CrossRef]
- Godfraind, T. Discovery and development of calcium channel blockers. Front. Pharmacol. 2017, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.L.; Iadecola, C.; Wang, G. New generations of dihydropyridines for treatment of hypertension. J. Geriatr. Cardiol. 2017, 14, 67–72. [Google Scholar] [CrossRef]
- Malhi, D.S.; Kaur, M.; Sohal, H.S. Effect of Substitutions on 1, 4-Dihdropyridines to Achieve Potential Anti-Microbial Drugs: A Review. ChemistrySelect 2019, 4, 11321–11336. [Google Scholar] [CrossRef]
- Khot, S.; Auti, P.B.; Khedkar, S.A. Diversified Synthetic Pathway of 1,4-Dihydropyridines: A Class of Pharmacologically Important Molecules. Mini-Rev. Med. Chem. 2020, 21, 135–149. [Google Scholar] [CrossRef]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Apsite, G.; Timofejeva, I.; Vezane, A.; Vigante, B.; Rucins, M.; Sobolev, A.; Plotniece, M.; Pajuste, K.; Kozlovska, T.; Plotniece, A. Synthesis and comparative evaluation of novel cationic amphiphile C12-Man-Q as an efficient DNA delivery agent in vitro. Molecules 2018, 23, 1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrichenko, O.; Plotniece, A.; Pajuste, K.; Rucins, M.; Dimitrijevs, P.; Sobolev, A.; Sprugis, E.; Cēbers, A. Evaluation of physicochemical properties of amphiphilic 1,4-dihydropyridines and preparation of magnetoliposomes. Nanomaterials 2021, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Stater, E.P.; Sonay, A.Y.; Hart, C.; Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 2021, 16, 1180–1194. [Google Scholar] [CrossRef]
- Rucins, M.; Dimitrijevs, P.; Pajuste, K.; Petrichenko, O.; Jackevica, L.; Gulbe, A.; Kibilda, S.; Smits, K.; Plotniece, M.; Tirzite, D.; et al. Contribution of molecular structure to self-assembling and biological properties of bifunctional lipid-like 4-(N-alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics 2019, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.G.; Rajani, D.P.; Patel, H.M. Green approach for synthesis of bioactive Hantzsch 1,4-dihydropyridine derivatives based on thiophene moiety via multicomponent reaction. R. Soc. Open Sci. 2017, 4, 170006. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Cao, S.; Wu, J.; Zhang, J.; Li, H.; Liu, N.; Qian, X. A revisit to the Hantzsch reaction: Unexpected products beyond 1,4-dihydropyridines. Green Chem. 2009, 11, 1414. [Google Scholar] [CrossRef]
- Wan, J.P.; Liu, Y. Recent advances in new multicomponent synthesis of structurally diversified 1,4-dihydropyridines. RSC Adv. 2012, 2, 9763–9777. [Google Scholar] [CrossRef]
- Comins, D.L.; Higuchi, K.; Young, D.W. Dihydropyridine preparation and application in the synthesis of pyridine derivatives. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2013; Volume 110, pp. 175–235. [Google Scholar]
- Magoo, D.; Aggarwal, K.; Gupta, S.; Meena, K. Enamines and their variants as intermediates for synthesis of aza-heterocycles with applications in MCRs. Tetrahedron 2022, 103, 132545. [Google Scholar] [CrossRef]
- Balalaie, S.; Baoosi, L.; Tahoori, F.; Rominger, F.; Bijanzadeh, H.R. Synthesis of polysubstituted 1,4-dihydropyridines via three-component reaction. Tetrahedron 2013, 69, 738–743. [Google Scholar] [CrossRef]
- Filipan-Litvić, M.; Litvić, M.; Cepanec, I.; Vinković, V. Hantzsch synthesis of 2,6-dimethyl-3,5-dimethoxycarbonyl-4-(o-methoxyphenyl)-1,4-dihydropyridine; a novel cyclisation leading to an unusual formation of 1-amino-2-methoxycarbonyl-3,5-bis(o-methoxyphenyl)-4-oxa- cyclohexan-1-ene. Molecules 2007, 12, 2546–2558. [Google Scholar] [CrossRef] [Green Version]
- Pajuste, K.; Plotniece, A.; Kore, K.; Intenberga, L.; Cekavicus, B.; Kaldre, D.; Duburs, G.; Sobolev, A. Use of pyridinium ionic liquids as catalysts for the synthesis of 3,5-bis(dodecyloxycarbonyl)-1,4-dihydropyridine derivative. Cent. Eur. J. Chem. 2011, 9, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Skarstin’sh, I.P.; Kastron, V.V.; Dubur, G.Y.; Mazheika, I.B.; Liepin’sh, E.E. Bromination of 2,6-dimethyl-3,5-dimethoxycarbonyl-4-(2′-difluoromethoxyphenyl)-1,4-dihydropyridine (foridone). Chem. Heterocycl. Compd. 1989, 25, 791–795. [Google Scholar] [CrossRef]
- Rucins, M.; Pajuste, K.; Sobolev, A.; Plotniece, M.; Pikun, N.; Pajuste, K.; Plotniece, A. Data for the synthesis and characterisation of 2,6-di(bromomethyl)-3,5-bis(alkoxycarbonyl)-4-aryl-1,4-dihydropyridines as important intermediates for synthesis of amphiphilic 1,4-dihydropyridines. Data Br. 2020, 30, 105532. [Google Scholar] [CrossRef]
- Skrastin’sh, I.P.; Kastron, V.V.; Chekavichus, B.S.; Sausin’sh, A.E.; Zolotoyabko, R.M.; Dubur, G.Y. Bromination of 4-aryl-3,5-dialkoxycarbonyl-2,6-dimethyl-1,4-dihydropyridines. Chem. Heterocycl. Compd. 1991, 27, 989–994. [Google Scholar] [CrossRef]
- Ponde, D.E.; Deshpande, V.H.; Bulbule, V.J.; Sudalai, A.; Gajare, A.S. Selective Catalytic Transesterification, Transthiolesterification, and Protection of Carbonyl Compounds over Natural Kaolinitic Clay. J. Org. Chem. 1998, 63, 1058–1063. [Google Scholar] [CrossRef]
- Rucins, M.; Plotniece, A.; Bernotiene, E.; Tsai, W.-B.; Sobolev, A. Recent approaches to chiral 1,4-dihydropyridines and their fused analogues. Catalysts 2020, 10, 1019. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, R.; Du, C.; Li, L. Betainium-based ionic liquids catalyzed multicomponent Hantzsch reactions for the efficient synthesis of acridinediones. RSC Adv. 2017, 7, 6679–6684. [Google Scholar] [CrossRef] [Green Version]
- Moheiseni, F.; Reza Kiasat, A.; Badri, R. Synthesis, Characterization and Application of β-Cyclodextrin/Imidazolium Based Dicationic Ionic Liquid Supported on Silica Gel as a Novel Catalyst in Hantzsch Condensation Reaction. Polycycl. Aromat. Compd. 2021, 41, 1094–1106. [Google Scholar] [CrossRef]
- Jassem, A.M.; Almashal, F.A.K.; Mohammed, M.Q.; Jabir, H.A.S. A catalytic and green method for one-pot synthesis of new Hantzsch 1,4-dihydropyridines. SN Appl. Sci. 2020, 2, 359. [Google Scholar] [CrossRef] [Green Version]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).