2-(4-(Dimethylamino)phenyl)-3,3-difluoro-4,6-diphenyl-3,4-dihydro-1,2,4,5,3-tetrazaborinin-2-ium-3-ide
Abstract
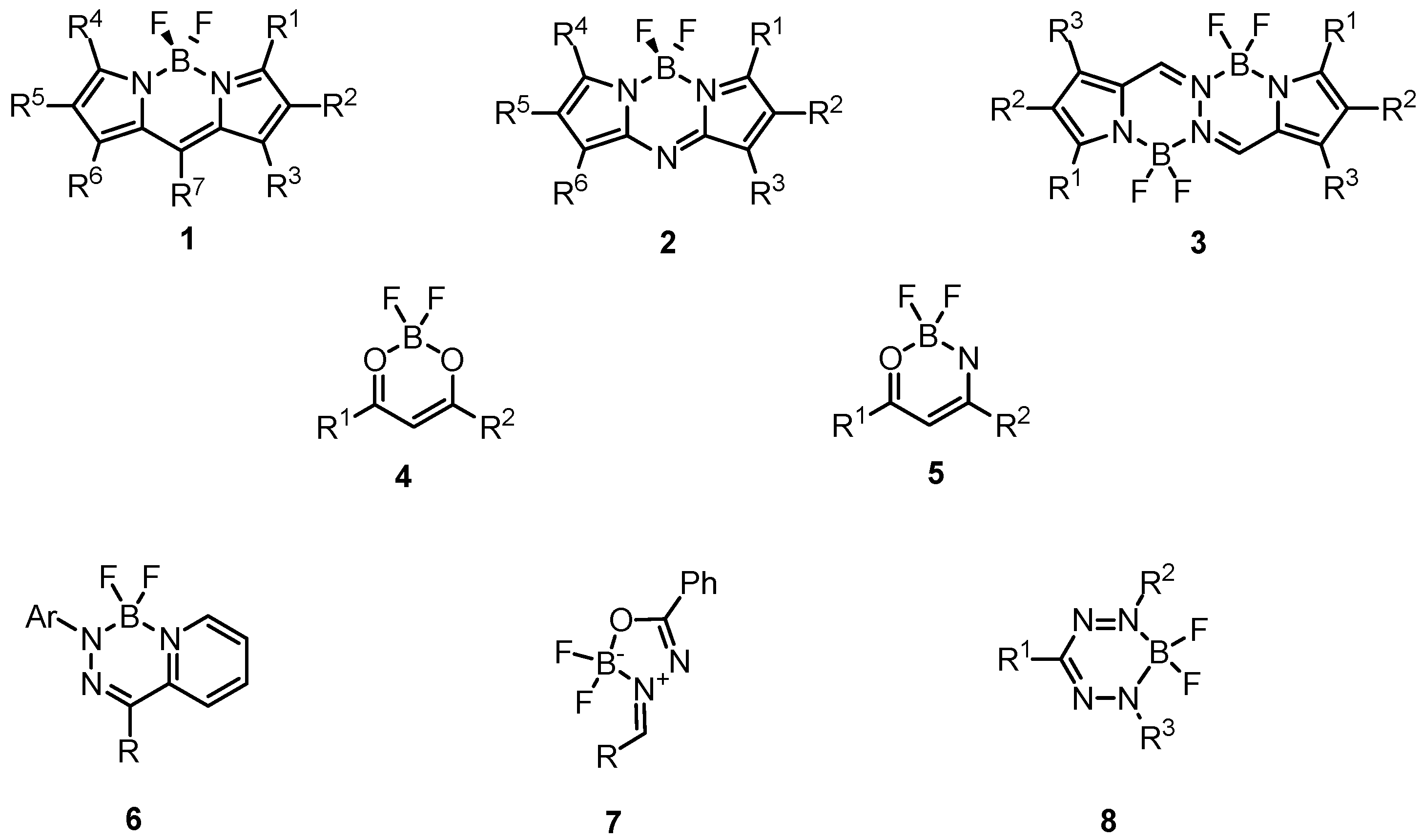
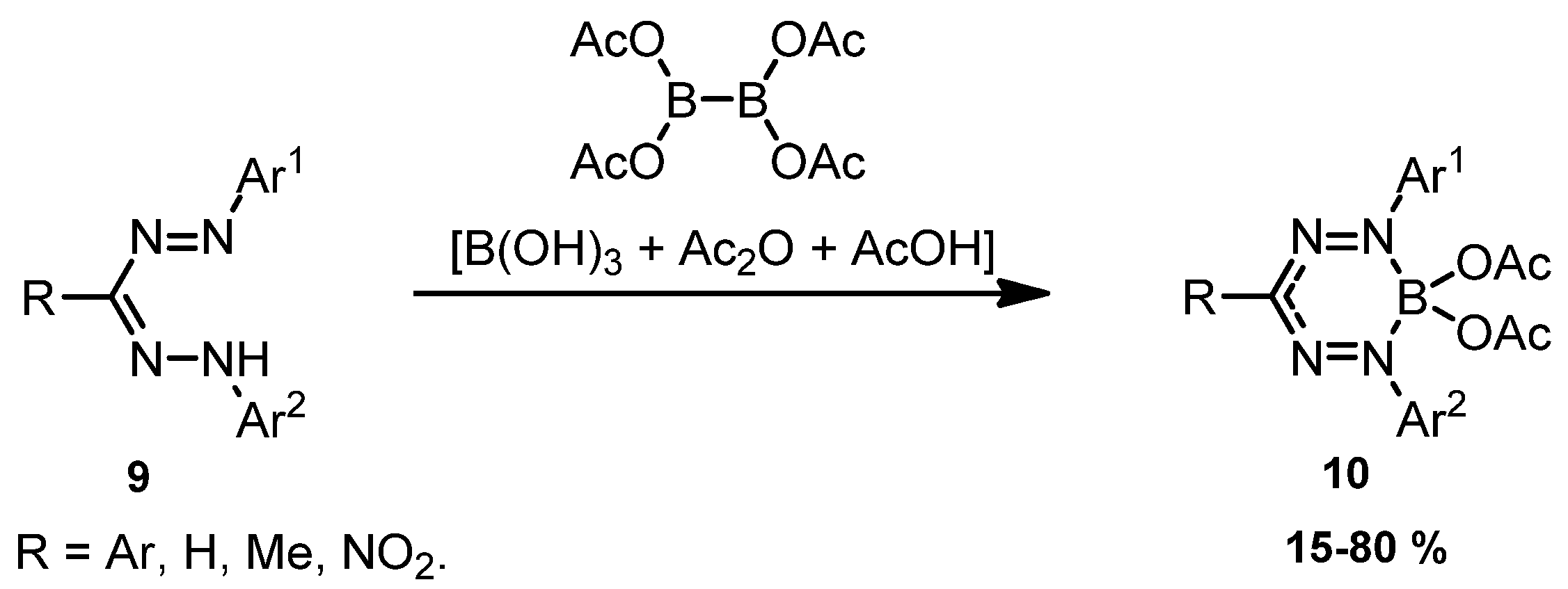
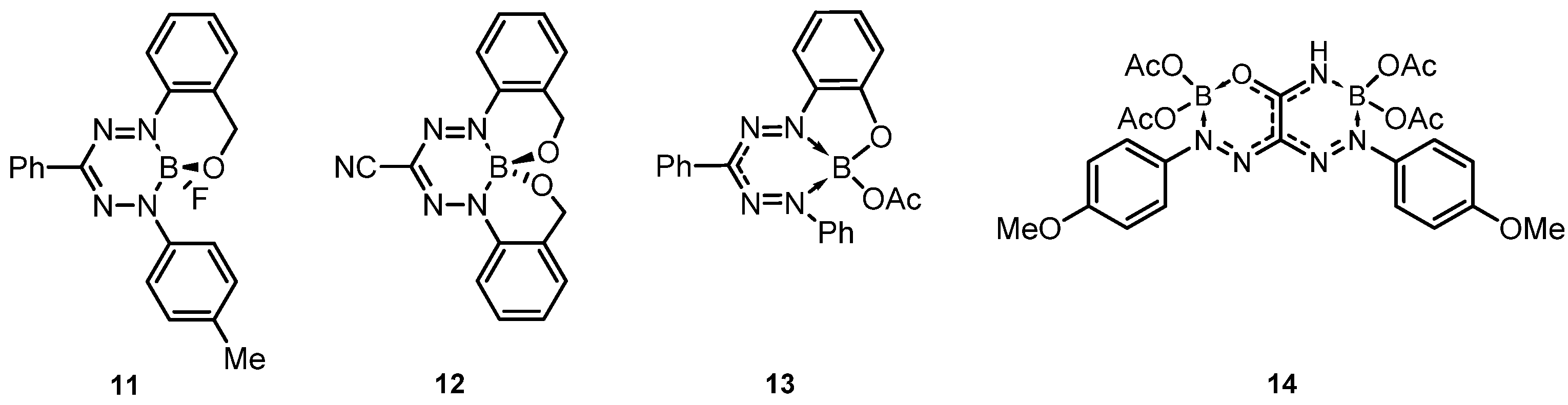
:1. Introduction
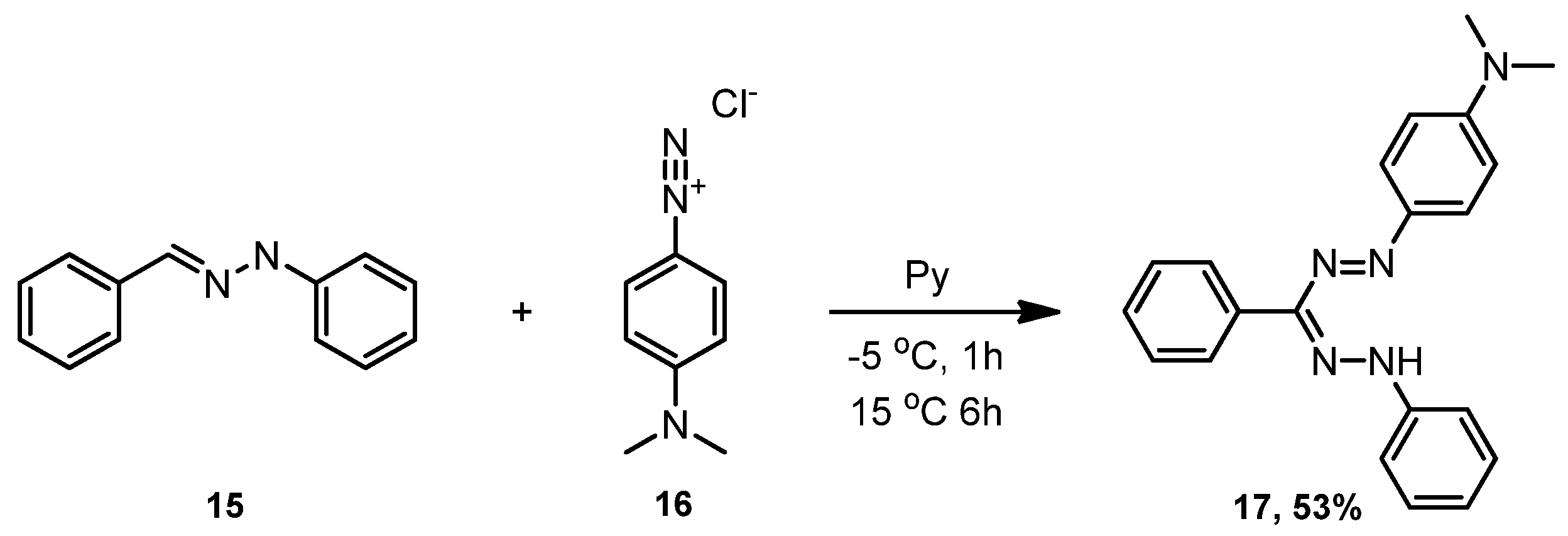
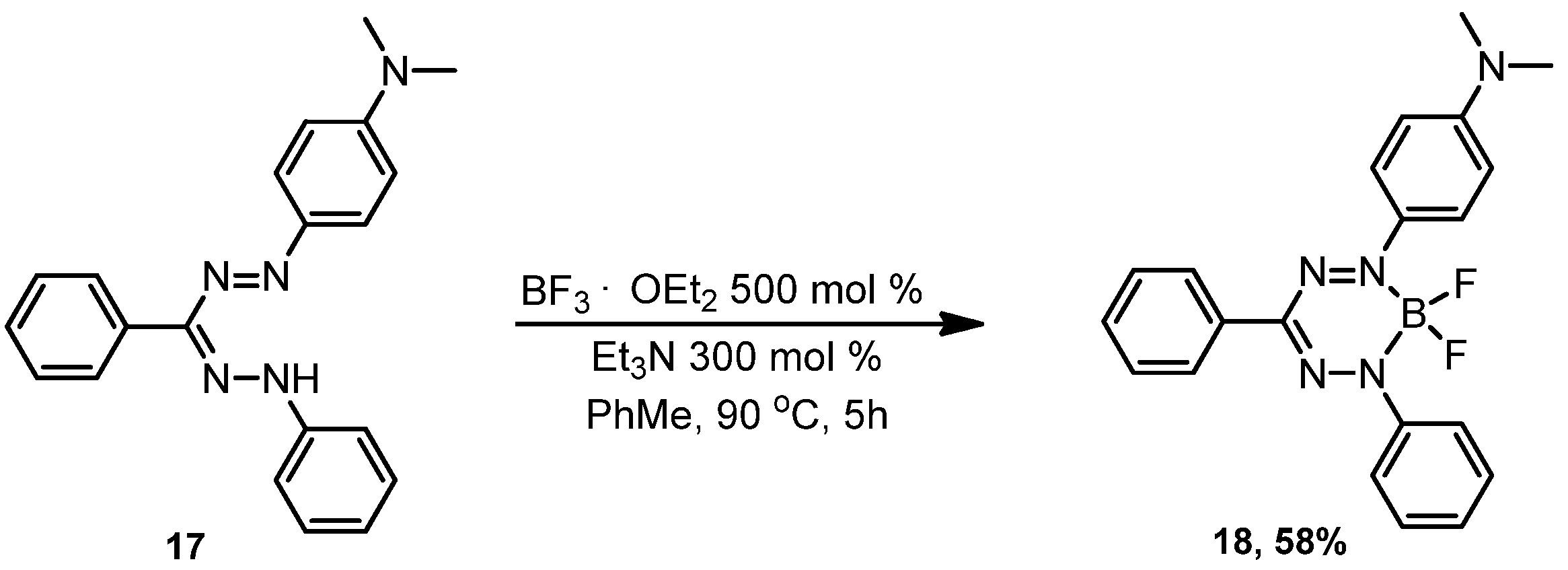
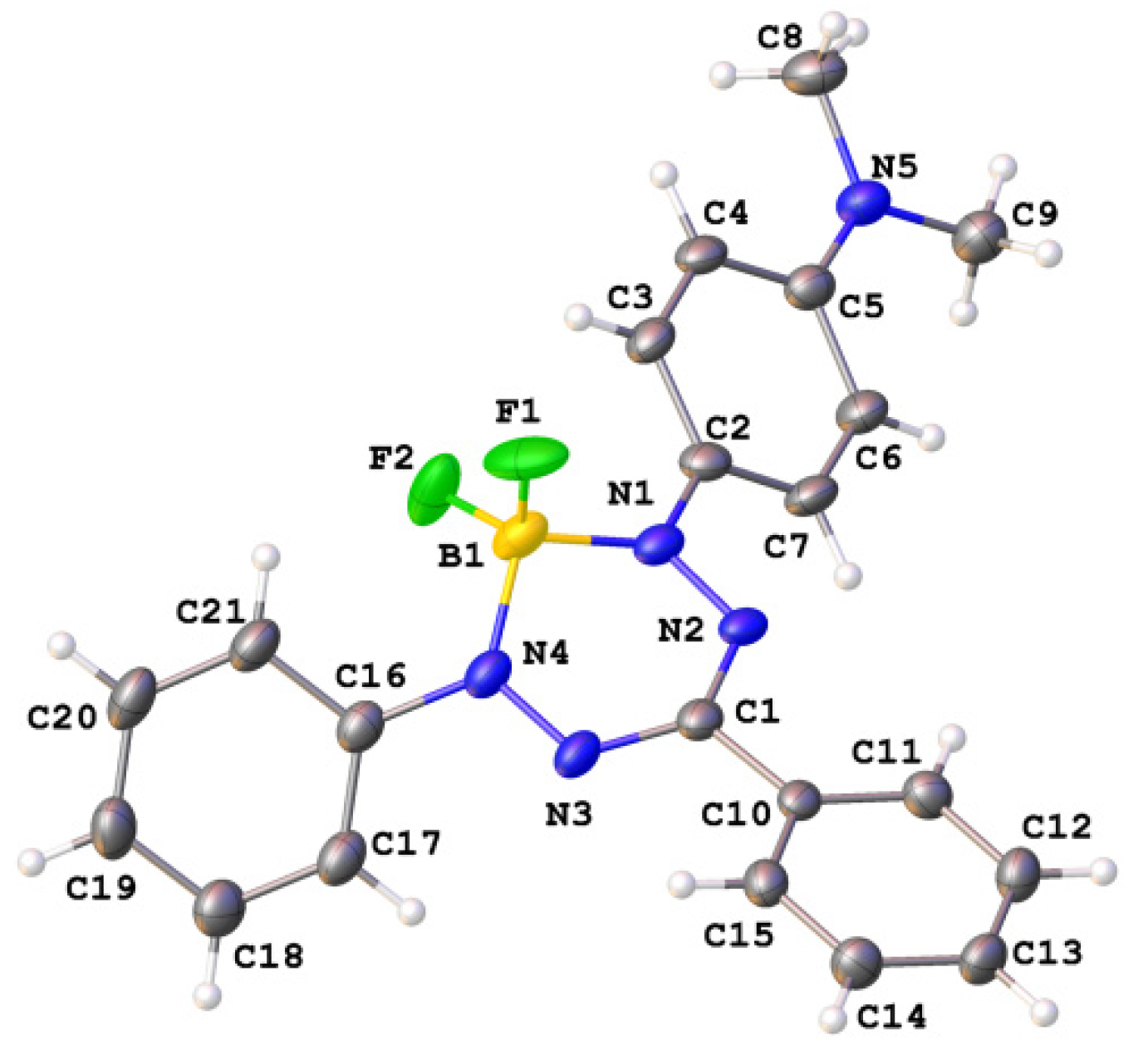
2. Results and Discussion
3. Materials and Methods
3.1. 1-(4-(Dimethylamino)phenyl)-3,5-diphenylformazan (17)
3.2. 2-(4-(Dimethylamino)phenyl)-3,3-difluoro-4,6-diphenyl-3,4-dihydro-1,2,4,5,3-tetrazaborinin-2-ium-3-ide (18)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhang, H.; Chen, S.; Liu, Y. Fluorescein-inspired near-infrared chemodosimeter for luminescence bioimaging. Curr. Med. Chem. 2019, 26, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, D.A.; Fares, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Ran, X.; Tang, H.; Cao, D. Recent progress on reaction-based BODIPY probes for anion detection. Dyes Pigm. 2020, 172, 107857. [Google Scholar] [CrossRef]
- Mellerup, S.K.; Wang, S. Boron-based stimuli responsive materials. Chem. Soc. Rev. 2019, 48, 3537–3549. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Wang, Y. Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs). Chem. Soc. Rev. 2013, 42, 8416–8433. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent advances of BODIPY based derivatives for optoelectronic applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-based molecules, a platform for photonic and solar cells. Molecules 2020, 26, 153. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Shamova, L.I.; Zatsikha, Y.V.; Nemykin, V.N. Synthesis pathways for the preparation of the BODIPY analogues: Aza-BODIPYs, BOPHYs and some other pyrrole-based acyclic chromophores. Dalton Trans. 2021, 50, 1569–1593. [Google Scholar] [CrossRef] [PubMed]
- Bismillah, A.N.; Aprahamian, I. Fundamental studies to emerging applications of pyrrole-BF2(BOPHY) fluorophores. Chem. Soc. Rev. 2021, 50, 5631–5649. [Google Scholar] [CrossRef]
- Collot, M. Recent advances in dioxaborine-based fluorescent materials for bioimaging applications. Mater. Horiz. 2021, 8, 501–514. [Google Scholar] [CrossRef]
- Fedorenko, E.V.; Mirochnik, A.G.; Beloliptsev, A.Y.; Svistunova, I.V.; Tretyakova, G.O. Design, synthesis, and crystallization-induced emission of boron difluorides β-ketoiminates. ChemPlusChem 2018, 83, 117–127. [Google Scholar] [CrossRef]
- Cappello, D.; Therien, D.A.B.; Staroverov, V.N.; Lagugné-Labarthet, F.; Gilroy, J.B. Optoelectronic, aggregation, and redox properties of double-rotor boron difluoride hydrazone dyes. Chem. Eur. J. 2019, 25, 5994–6006. [Google Scholar] [CrossRef] [Green Version]
- Dilman, A.D.; Arkhipov, D.E.; Levin, V.V.; Belyakov, P.A.; Korlyukov, A.A.; Struchkova, M.I.; Tartakovsky, V.A. Trifluoromethylation of N-benzoylhydrazones. J. Org. Chem. 2008, 73, 5643–5646. [Google Scholar] [CrossRef]
- Gilroy, J.B.; Otten, E. Formazanate coordination compounds: Synthesis, reactivity, and applications. Chem. Soc. Rev. 2020, 49, 85–113. [Google Scholar] [CrossRef] [Green Version]
- Acarbay, M. Über Formazyl-Verbindungen, XI. Über die reaktion von Formazyl-Verbindungen mit Bortrifluorid-ätherat. Justus Liebigs Ann. Chem. 1964, 677, 127–128. [Google Scholar] [CrossRef]
- Stepanov, B.I.; Avramenko, G.V. Boron—nitrogen compounds I. Reactions of triarylformazans with diboron tetraacetate. Synthesis of boratetrazines. J. Gen. Chem. (USSR) 1980, 50, 292–295. [Google Scholar]
- Gilroy, J.B.; Ferguson, M.J.; McDonald, R.; Patrick, B.O.; Hicks, R.G. Formazans as β-diketiminate analogues. Structural characterization of boratatetrazines and their reduction to borataverdazyl radical anions. Chem. Commun. 2007, 2, 126–128. [Google Scholar] [CrossRef]
- Barbon, S.M.; Price, J.T.; Reinkeluers, P.A.; Gilroy, J.B. Substituent-dependent optical and electrochemical properties of triarylformazanate boron difluoride complexes. Inorg. Chem. 2014, 53, 10585–10593. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.M.; Reinkeluers, P.A.; Price, J.T.; Staroverov, V.N.; Gilroy, J.B. Structurally tunable 3-cyanoformazanate boron difluoride dyes. Chem. Eur. J. 2014, 20, 11340–11344. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.M.; Staroverov, V.N.; Gilroy, J.B. Effect of extended π conjugation on the spectroscopic and electrochemical properties of boron difluoride formazanate complexes. J. Org. Chem. 2015, 80, 5226–5235. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.; Agrawal, A.R.; Ghosh, N.G.; Karmakar, H.S.; Das, S.; Kumar, N.R.; Banewar, V.W.; Zade, S.S. Boron difluoride formazanates with thiophene and 3,4-ethylenedioxythiophene capping and their electrochemical polymerization. Dalton Trans. 2020, 49, 13202–13206. [Google Scholar] [CrossRef]
- Dhindsa, J.S.; Buguis, F.L.; Anghel, M.; Gilroy, J.B. Band gap engineering in acceptor-donor-acceptor boron difluoride formazanates. J. Org. Chem. 2021, 86, 12064–12074. [Google Scholar] [CrossRef] [PubMed]
- Maar, R.R.; Katzman, B.D.; Boyle, P.D.; Staroverov, V.N.; Gilroy, J.B. Cationic boron formazanate dyes. Angew. Chem. Int. Ed. 2021, 60, 5152–5156. [Google Scholar] [CrossRef]
- Van Belois, A.; Maar, R.R.; Workentin, M.S.; Gilroy, J.B. Dialkynylborane complexes of formazanate ligands: Synthesis, electronic properties, and reactivity. Inorg. Chem. 2019, 58, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Katzman, B.D.; Maar, R.R.; Cappello, D.; Sattler, M.O.; Boyle, P.D.; Staroverov, V.N.; Gilroy, J.B. A strongly Lewis-acidic and fluorescent borenium cation supported by a tridentate formazanate ligand. Chem. Commun. 2021, 57, 9530–9533. [Google Scholar] [CrossRef]
- Barbon, S.M.; Staroverov, V.N.; Gilroy, J.B. Structurally diverse Boron–Nitrogen heterocycles from an N2O23− formazanate ligand. Angew. Chem. Int. Ed. 2017, 56, 8173–8177. [Google Scholar] [CrossRef]
- Stepanov, B.I.; Avramenko, G.V.; Khamud, S.; Mustafaeva, S.I. Boratetrazines. J. Gen. Chem. (USSR) 1986, 56, 339–341. [Google Scholar]
- Bezuglaya, Z.V.; Naser, M.; Avramenko, G.V.; Stepanov, B.I. Influence of substitution on the path of complexation of 1, 5-diarylformazan-3-carboxylic acid amides with diobortetraacetate. J. Gen. Chem. (USSR) 1991, 61, 1758–1759. [Google Scholar]
- Maar, R.R.; Zhang, R.; Stephens, D.G.; Ding, Z.; Gilroy, J.B. Near-infrared photoluminescence and electrochemiluminescence from a remarkably simple boron difluoride formazanate dye. Angew. Chem. Int. Ed. 2019, 58, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Petunin, P.V.; Martynko, E.A.; Trusova, M.E.; Kazantsev, M.S.; Rybalova, T.V.; Valiev, R.R.; Uvarov, M.N.; Mostovich, E.A.; Postnikov, P.S. Verdazyl radical building blocks: Synthesis, structure, and sonogashira cross-coupling reactions. Eur. J. Org. Chem. 2018, 34, 4802–4811. [Google Scholar] [CrossRef]
- Kostryukov, S.G.; Balandina, A.V.; Kozlov, A.S.; Kraynov, E.V.; Pryanichnikova, M.K.; Chernyaeva, O.Y.; Akhmatova, A.A.; Lukshina, Y.I. Synthesis and electrochemical properties of 2-(4-R1-phenyl)-6-(4-R2-phenyl)-4-phenyl-3,4-dihydro1,2,4,5-tetrazin-1(2H)-yls. Russ. J. Gen. Chem. 2020, 90, 341–351. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupanova, I.A.; Konshina, D.N.; Elkov, N.A.; Konshin, V.V. 2-(4-(Dimethylamino)phenyl)-3,3-difluoro-4,6-diphenyl-3,4-dihydro-1,2,4,5,3-tetrazaborinin-2-ium-3-ide. Molbank 2022, 2022, M1312. https://doi.org/10.3390/M1312
Lupanova IA, Konshina DN, Elkov NA, Konshin VV. 2-(4-(Dimethylamino)phenyl)-3,3-difluoro-4,6-diphenyl-3,4-dihydro-1,2,4,5,3-tetrazaborinin-2-ium-3-ide. Molbank. 2022; 2022(1):M1312. https://doi.org/10.3390/M1312
Chicago/Turabian StyleLupanova, Ida A., Dzhamilya N. Konshina, Nikita A. Elkov, and Valery V. Konshin. 2022. "2-(4-(Dimethylamino)phenyl)-3,3-difluoro-4,6-diphenyl-3,4-dihydro-1,2,4,5,3-tetrazaborinin-2-ium-3-ide" Molbank 2022, no. 1: M1312. https://doi.org/10.3390/M1312
APA StyleLupanova, I. A., Konshina, D. N., Elkov, N. A., & Konshin, V. V. (2022). 2-(4-(Dimethylamino)phenyl)-3,3-difluoro-4,6-diphenyl-3,4-dihydro-1,2,4,5,3-tetrazaborinin-2-ium-3-ide. Molbank, 2022(1), M1312. https://doi.org/10.3390/M1312





