Abstract
2,6-Bis(benzo[c][1,2,5]thiadiazol-4-yl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophenes are of interest for the synthesis of molecules which can be employed in optoelectronic devices. In this communication, 7,7’-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole) was obtained by direct C–H cross-coupling of 4,7-dibromobenzo[c][1,2,5]thiadiazole with 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene in the presence of palladium(II)acetate and potassium pivalate. The structure of newly synthesized compound was established by means of elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR, IR and UV spectroscopy.
1. Introduction
π-Conjugated molecules have been often used in organic optoelectronic devices such as organic light emitting diodes (OLEDs), organic field effect transistors (OFETs), organic solar cells (OSCs) and others [1,2]. Numerous combinations of donor, acceptor and π-spacer elements are employed to achieve the desired properties of the optoelectronic material by increasing polarizability and tuning the energy band gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) [3,4,5]. One of the interesting molecular intermediates 2,6-bis(benzo[c][1,2,5]thiadiazol-4-yl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophenes, which contain electron deficient moiety, for example benzo[c][1,2,5]thiadiazole, linked through a π-conjugated bridge, i.e., 4H-cyclopenta[2,1-b:3,4-b’]dithiophene, has been frequently used for the synthesis various polymer and oligomer materials [6,7,8,9,10,11,12,13].
From a synthetic point of view, the formation of 2,6-bis(benzo[c][1,2,5]thiadiazol-4-yl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophenes requires the formation of C–C bonds, as a rule, using cross-coupling reactions. In all the reactions described, the cross-coupling by Stille [6,7,8,9] and, less often, by Suzuki [10,11] was employed. Yields may vary from 30% to 98%. On the other hand, it is known that direct arylation can occur via the C–H bond without the need for organometallic intermediates. This method was proposed several decades ago by Ohta et al. [14] with the example of direct arylation of thiophenes with aryl halides. Recently, direct C–H arylation has been developed as a method for synthesis conjugated materials for use in organic electronics from simple building blocks [15,16,17,18]. This method offers shorter and cheaper reaction paths for intermediates and the final synthesis of materials, and also leads to less toxic waste compared to classical cross-coupling protocols. Herein, we report the synthesis of 7,7’-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole) by direct C–H cross-coupling of 4,7-dibromobenzo[c][1,2,5]thiadiazole with 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene.
2. Results and Discussion
We studied the Pd-catalyzed direct C–H arylation reaction of 4,7-dibromobenzo[c][1,2,5]thiadiazole 1 with 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene 2 and the most frequently used bases in this type of reactions—potassium and cesium pivalates (generated in situ from the corresponding carbonates and pivalic acid) and potassium acetate (Scheme 1, Table 1). The search for optimal conditions was carried out by varying the bases, solvents, and the reaction temperature. It was found that the reaction between 4,7-dibromobenzo[c][1,2,5]thiadiazole 1 and 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene 2 in the presence of palladium(II)acetate and potassium pivalate begins at temperatures above 60 °C; at a temperature of 40 °C, the starting compounds remain in the reaction mass (Table 1, Entry 1). When the reaction was carried out under heating (at 80 °C or higher) in DMF or DMAc, product 3 was isolated in a yield of 23–27% (Table 1, Entries 2–4). It has been shown that increasing the temperature or replacing potassium pivalate with cesium pivalate does not improve the yields of the target product. We assume that the low yields are the result of the oligomerization of product 3 and 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene 2.
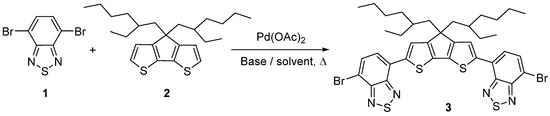
Scheme 1.
Synthesis of 7,7’-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole) 3.

Table 1.
Reaction of 4,7-dibromobenzo[c][1,2,5]thiadiazole 1 with 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene 2.
The structure of 7,7’-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole) 3 was confirmed by means of elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR, IR and UV spectroscopy.
3. Materials and Methods
4,7-Dibromobenzo[c][1,2,5]thiadiazole 1 [19] and 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene 2 [20] were prepared according to the published methods. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin ElmerInc., Waltham, MA, USA). The melting point was determined on a Kofler hot-stage apparatus and is uncorrected. 1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300 and 75 MHz) in CDCl3 solution with TMS as the standard. J values are given in Hz. The IR spectrum was measured with a Bruker “Alpha-T” instrument in KBr pellet. The high-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (Supplementary Materials). The solution’s UV-visible absorption spectra were recorded using an OKB Spektr SF-2000 UV/Vis/NIR spectrophotometer controlled with SF-2000 software. The sample was measured in a 1 cm quartz cell at room temperature with 4.8 × 10−5 mol/mL concentration in CH2Cl2.
Synthesis of 7,7’-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole) 3 (Supplementary Materials).
A mixture of 4,7-dibromobenzo[c][1,2,5]thiadiazole 1 (880 mg, 3.02 mmol), pivalic acid (520 mg, 4.33 mmol) and potassium carbonate (800 mg, 5.79 mmol) in dry DMF (20 mL) was degassed by argon for 20 min, and Pd(OAc)2 (50 mg, 0.23 mmol) was added. The mixture was stirred at room temperature for 10 min and then the solution of 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene 2 (580 mg, 1.44 mmol) in DMF (7 mL) was dropwise added during 0.5 h. Reaction mixture was heated to 80 °C for 4 h. On completion, the mixture was poured into water and extracted with CH2Cl2 (3 × 10 mL). The combined organic phases were washed with water (5 × 25 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (silica gel Merck 60, eluent-petroleum ether). Yield 322 mg (27%), purple solid with mp = 46–47 °C, Rf = 0.71 (petroleum ether–ethyl acetate, 10:1, v/v). IR spectrum, ν, cm−1: 3499, 3438, 2954, 2921 and 2853 (C–H), 1631, 1575, 1498, 1395, 1227, 1183, 823. 1H NMR (ppm, CDCl3): δ 8.10 (t, J = 4.6, 2H, the ratio was 1:2:1 due to racemic 2-ethylhexyl groups [21,22]), 7.84 (d, J = 7.8, 2H), 7.70 (d, J = 7.8, 2H), 2.10–1.99 (m, 4H), 1.07–0.93 (m, 18H), 0.68–0.61 (m, 12H). 13C NMR (ppm): δ 159.5, 153.9, 151.6, 139.4, 132.4, 128.0, 124.2, 123.6, 123.4, 111.4, 54.4, 43.2, 35.4, 34.3, 28.6, 27.6, 22.8, 14.0, 10.8. HRMS (ESI-TOF), m/z: calcd for C37H40Br2N4S4 [M + H]+, 828.0473, found, 828.0478. UV-Vis (CH2Cl2, λmax, nm/logε): 364/3.75, 539/4.01. Anal. calcd. For C37H40Br2N4S4 (828.0473): C, 53.62; H, 4.83; N, 6.76. Found: C, 53.89; H, 4.97; N, 6.86%.
Supplementary Materials
The following are available online: copies of 1H, 13C NMR, IR and UV-Vis spectra for the compound 3.
Author Contributions
Analysis of experimental results and NMR data, E.A.K.; synthetic experiments and resources, N.S.G.; conceptualization, writing—review and editing supervision and project administration, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science of the Russian Federation, grant number FENU-2020-0019 (2020073GZ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.-C.; Wong, W.-Y. Recent progress of electronic materials based on 2,1,3-benzothiadiazole and its derivatives: Synthesis and their application in organic light-emitting diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- Kim, T.-D.; Lee, K.-S. D-π-A Conjugated molecules for optoelectronic applications. Macromol. Rapid Commun. 2015, 36, 943–958. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, X.; Xiao, M.; Tan, H.; Tao, Q.; Wang, Y.; Liu, Y.; Yang, R.; Zhu, W. Significantly improved photovoltaic performance of the triangular-spiral TPA(DPP–PN)3 by appending planar phenanthrene units into the molecular terminals. J. Mater. Chem. A 2015, 3, 886–893. [Google Scholar] [CrossRef]
- Bureš, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef] [Green Version]
- Welch, G.C.; Bazan, G.C. Lewis acid adducts of narrow band gap conjugated polymers. J. Am. Chem. Soc. 2011, 133, 4632–4644. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.W.; Henson, Z.B.; Du, J.; Vandenberg, C.A.; Bazan, G.C. Synthesis, characterization, and biological affinity of a near-infrared-emitting conjugated oligoelectrolyte. J. Am. Chem. Soc. 2014, 136, 3736–3739. [Google Scholar] [CrossRef]
- Tautz, R.; Da Como, E.; Wiebeler, C.; Soavi, G.; Dumsch, I.; Fröhlich, N.; Grancini, G.; Allard, S.; Scherf, U.; Cerullo, G.; et al. Charge photogeneration in donor−acceptor conjugated materials: Influence of excess excitation energy and chain length. J. Am. Chem. Soc. 2013, 135, 4282–4290. [Google Scholar] [CrossRef]
- Wang, K.; Firdaus, Y.; Babics, M.; Cruciani, F.; Saleem, Q.; El Labban, A.; Alamoudi, M.A.; Marszalek, T.; Pisula, W.; Laquai, F.; et al. π-Bridge-independent 2-(benzo[c][1,2,5]thiadiazol-4-ylmethylene)malononitrile-substituted nonfullerene acceptors for efficient bulk heterojunction solar cells. Chem. Mater. 2016, 28, 2200–2208. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Lin, T.; Wang, F.; He, C. Regiospecific protonation of organic chromophores. Phys. Chem. Chem. Phys. 2016, 18, 18758–18766. [Google Scholar] [CrossRef]
- Horie, M.; Kettle, J.; Yu, C.-Y.; Majewski, L.A.; Chang, S.-W.; Kirkpatrick, J.; Tuladhar, S.M.; Nelson, J.; Saunders, B.R.; Turner, M.L. Cyclopentadithiophene-benzothiadiazole oligomers and polymers; synthesis, characterisation, field-effect transistor and photovoltaic characteristics. J. Mater. Chem. 2012, 22, 381–389. [Google Scholar] [CrossRef]
- Hou, J.; Chen, T.L.; Zhang, S.; Chen, H.-Y.; Yang, Y. Poly[4,4-bis(2-ethylhexyl)cyclopenta[2,1-b;3,4-b′]dithiophene-2,6-diyl-alt-2,1,3-benzoselenadiazole-4,7-diyl], a new low band gap polymer in polymer solar cells. J. Phys. Chem. C 2009, 113, 1601–1605. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef] [Green Version]
- Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Palladium-catalyzed arylation of furan, thiophene, benzo[b]furan and benzo[b]thiophene. Heterocycles 1990, 31, 1951–1958. [Google Scholar] [CrossRef]
- Panigrahi, P.; Mallick, M.K.; Mohanty, S.; Nayak, S.K.; Palai, A.K. EDOT based terpolymers: Facile synthesis via direct c-h arylation and barrier layer in dye sensitized solar cells. ChemistrySelect 2020, 5, 8674–8678. [Google Scholar] [CrossRef]
- Yemene, A.E.; Venkatraman, V.; Almenningen, D.M.; Hoff, B.H.; Gautun, O.R. Synthesis of novel 3,6-dithienyl diketopyrrolopyrrole dyes by direct C-H arylation. Molecules 2020, 25, 2349. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, C.; Babin, V.; Jouanne, M.; Slimani, I.; Berhault, Y.; Legay, R.; Sopková-de Oliveira Santos, J.; Rault, S.; Voisin-Chiret, A.S. Sequential one pot double C-H heteroarylation of thiophene using bromopyridines to synthesize unsymmetrical 2,5-bipyridylthiophenes. Tetrahedron 2017, 73, 5509–5516. [Google Scholar] [CrossRef]
- Sharma, B.; Alam, F.; Dutta, V.; Jacob, J. Synthesis and photovoltaic studies on novel fluorene based cross-conjugated donor-acceptor type polymers. Org. Electron. 2017, 40, 42–50. [Google Scholar] [CrossRef]
- Pilgram, K.; Zupan, M.; Skiles, R. Bromination of 2,1,3-benzothiadiazoles. J. Heterocycl. Chem. 1970, 7, 629. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsieh, C.-H.; Dubosc, M.; Cheng, Y.-J.; Hsu, C.-S. Synthesis and characterization of bridged bithiophene-based conjugated polymers for photovoltaic applications: Acceptor strength and ternary blends. Macromolecules 2010, 43, 697–708. [Google Scholar] [CrossRef]
- Chang, S.-W.; Horie, M. A donor–acceptor conjugated block copolymer of poly(arylenevinylene)s by ring-opening metathesis polymerization. Chem. Commun. 2015, 51, 9113–9116. [Google Scholar] [CrossRef] [PubMed]
- Mone, M.; Yang, K.; Murto, P.; Zhang, F.; Wang, E. Low-gap zinc porphyrin as an efficient dopant for photomultiplication type photodetectors. Chem. Commun. 2020, 56, 12769–12772. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).