7,7’-(4,4-Bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole)
Abstract
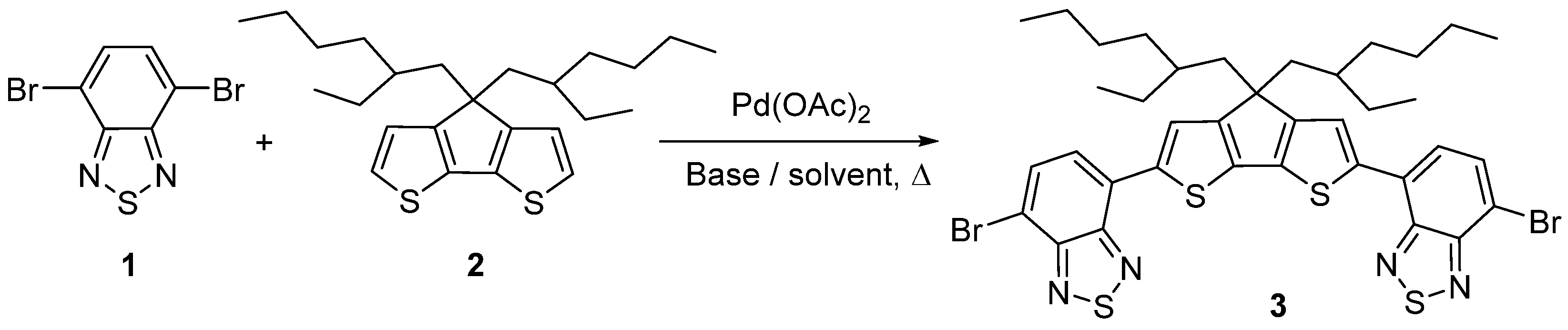
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.-C.; Wong, W.-Y. Recent progress of electronic materials based on 2,1,3-benzothiadiazole and its derivatives: Synthesis and their application in organic light-emitting diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- Kim, T.-D.; Lee, K.-S. D-π-A Conjugated molecules for optoelectronic applications. Macromol. Rapid Commun. 2015, 36, 943–958. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, X.; Xiao, M.; Tan, H.; Tao, Q.; Wang, Y.; Liu, Y.; Yang, R.; Zhu, W. Significantly improved photovoltaic performance of the triangular-spiral TPA(DPP–PN)3 by appending planar phenanthrene units into the molecular terminals. J. Mater. Chem. A 2015, 3, 886–893. [Google Scholar] [CrossRef]
- Bureš, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef] [Green Version]
- Welch, G.C.; Bazan, G.C. Lewis acid adducts of narrow band gap conjugated polymers. J. Am. Chem. Soc. 2011, 133, 4632–4644. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.W.; Henson, Z.B.; Du, J.; Vandenberg, C.A.; Bazan, G.C. Synthesis, characterization, and biological affinity of a near-infrared-emitting conjugated oligoelectrolyte. J. Am. Chem. Soc. 2014, 136, 3736–3739. [Google Scholar] [CrossRef]
- Tautz, R.; Da Como, E.; Wiebeler, C.; Soavi, G.; Dumsch, I.; Fröhlich, N.; Grancini, G.; Allard, S.; Scherf, U.; Cerullo, G.; et al. Charge photogeneration in donor−acceptor conjugated materials: Influence of excess excitation energy and chain length. J. Am. Chem. Soc. 2013, 135, 4282–4290. [Google Scholar] [CrossRef]
- Wang, K.; Firdaus, Y.; Babics, M.; Cruciani, F.; Saleem, Q.; El Labban, A.; Alamoudi, M.A.; Marszalek, T.; Pisula, W.; Laquai, F.; et al. π-Bridge-independent 2-(benzo[c][1,2,5]thiadiazol-4-ylmethylene)malononitrile-substituted nonfullerene acceptors for efficient bulk heterojunction solar cells. Chem. Mater. 2016, 28, 2200–2208. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Lin, T.; Wang, F.; He, C. Regiospecific protonation of organic chromophores. Phys. Chem. Chem. Phys. 2016, 18, 18758–18766. [Google Scholar] [CrossRef]
- Horie, M.; Kettle, J.; Yu, C.-Y.; Majewski, L.A.; Chang, S.-W.; Kirkpatrick, J.; Tuladhar, S.M.; Nelson, J.; Saunders, B.R.; Turner, M.L. Cyclopentadithiophene-benzothiadiazole oligomers and polymers; synthesis, characterisation, field-effect transistor and photovoltaic characteristics. J. Mater. Chem. 2012, 22, 381–389. [Google Scholar] [CrossRef]
- Hou, J.; Chen, T.L.; Zhang, S.; Chen, H.-Y.; Yang, Y. Poly[4,4-bis(2-ethylhexyl)cyclopenta[2,1-b;3,4-b′]dithiophene-2,6-diyl-alt-2,1,3-benzoselenadiazole-4,7-diyl], a new low band gap polymer in polymer solar cells. J. Phys. Chem. C 2009, 113, 1601–1605. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef] [Green Version]
- Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Palladium-catalyzed arylation of furan, thiophene, benzo[b]furan and benzo[b]thiophene. Heterocycles 1990, 31, 1951–1958. [Google Scholar] [CrossRef]
- Panigrahi, P.; Mallick, M.K.; Mohanty, S.; Nayak, S.K.; Palai, A.K. EDOT based terpolymers: Facile synthesis via direct c-h arylation and barrier layer in dye sensitized solar cells. ChemistrySelect 2020, 5, 8674–8678. [Google Scholar] [CrossRef]
- Yemene, A.E.; Venkatraman, V.; Almenningen, D.M.; Hoff, B.H.; Gautun, O.R. Synthesis of novel 3,6-dithienyl diketopyrrolopyrrole dyes by direct C-H arylation. Molecules 2020, 25, 2349. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, C.; Babin, V.; Jouanne, M.; Slimani, I.; Berhault, Y.; Legay, R.; Sopková-de Oliveira Santos, J.; Rault, S.; Voisin-Chiret, A.S. Sequential one pot double C-H heteroarylation of thiophene using bromopyridines to synthesize unsymmetrical 2,5-bipyridylthiophenes. Tetrahedron 2017, 73, 5509–5516. [Google Scholar] [CrossRef]
- Sharma, B.; Alam, F.; Dutta, V.; Jacob, J. Synthesis and photovoltaic studies on novel fluorene based cross-conjugated donor-acceptor type polymers. Org. Electron. 2017, 40, 42–50. [Google Scholar] [CrossRef]
- Pilgram, K.; Zupan, M.; Skiles, R. Bromination of 2,1,3-benzothiadiazoles. J. Heterocycl. Chem. 1970, 7, 629. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsieh, C.-H.; Dubosc, M.; Cheng, Y.-J.; Hsu, C.-S. Synthesis and characterization of bridged bithiophene-based conjugated polymers for photovoltaic applications: Acceptor strength and ternary blends. Macromolecules 2010, 43, 697–708. [Google Scholar] [CrossRef]
- Chang, S.-W.; Horie, M. A donor–acceptor conjugated block copolymer of poly(arylenevinylene)s by ring-opening metathesis polymerization. Chem. Commun. 2015, 51, 9113–9116. [Google Scholar] [CrossRef] [PubMed]
- Mone, M.; Yang, K.; Murto, P.; Zhang, F.; Wang, E. Low-gap zinc porphyrin as an efficient dopant for photomultiplication type photodetectors. Chem. Commun. 2020, 56, 12769–12772. [Google Scholar] [CrossRef] [PubMed]
| Entry | Solvent | Base | T, °C | Time, h | Yield of 3, % |
|---|---|---|---|---|---|
| 1 | DMF | K2CO3/PivOH | 40 | 10 | 0 |
| 2 | DMF | K2CO3/PivOH | 80 | 4 | 27 |
| 3 | DMAc | AcOK | 155 | 4 | 25 |
| 4 | DMAc | Cs2CO3/PivOH | 155 | 4 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudim, N.S.; Knyazeva, E.A.; Rakitin, O.A. 7,7’-(4,4-Bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole). Molbank 2022, 2022, M1310. https://doi.org/10.3390/M1310
Gudim NS, Knyazeva EA, Rakitin OA. 7,7’-(4,4-Bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole). Molbank. 2022; 2022(1):M1310. https://doi.org/10.3390/M1310
Chicago/Turabian StyleGudim, Nikita S., Ekaterina A. Knyazeva, and Oleg A. Rakitin. 2022. "7,7’-(4,4-Bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole)" Molbank 2022, no. 1: M1310. https://doi.org/10.3390/M1310
APA StyleGudim, N. S., Knyazeva, E. A., & Rakitin, O. A. (2022). 7,7’-(4,4-Bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl)bis(4-bromobenzo[c][1,2,5]thiadiazole). Molbank, 2022(1), M1310. https://doi.org/10.3390/M1310







