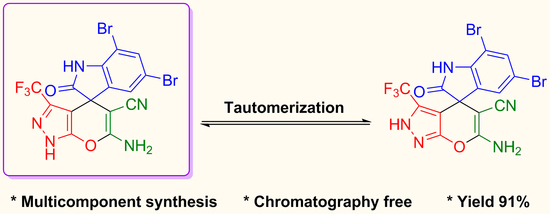
6′-Amino-5,7-dibromo-2-oxo-3′-(trifluoromethyl)-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile
Abstract
1. Introduction
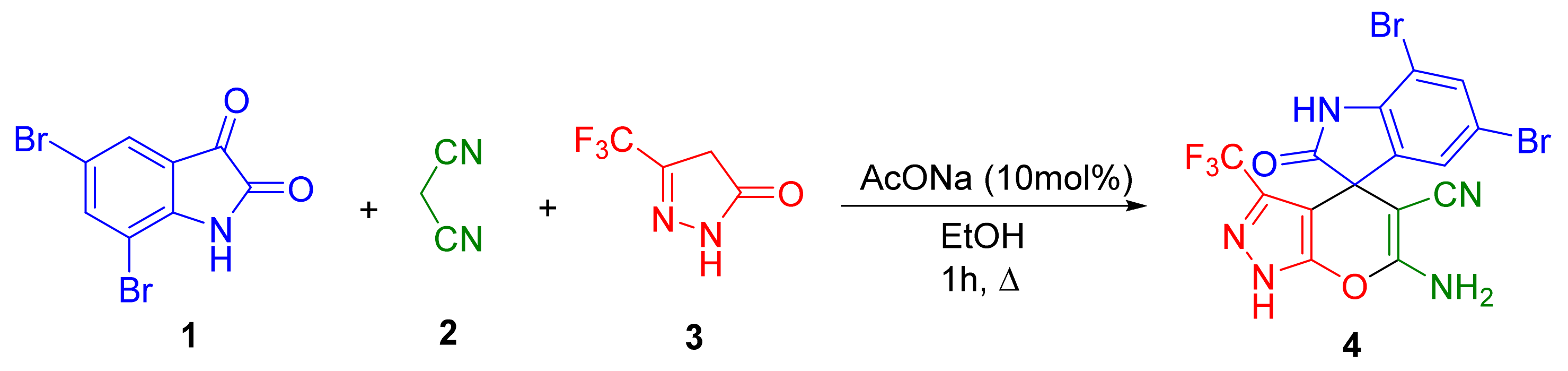
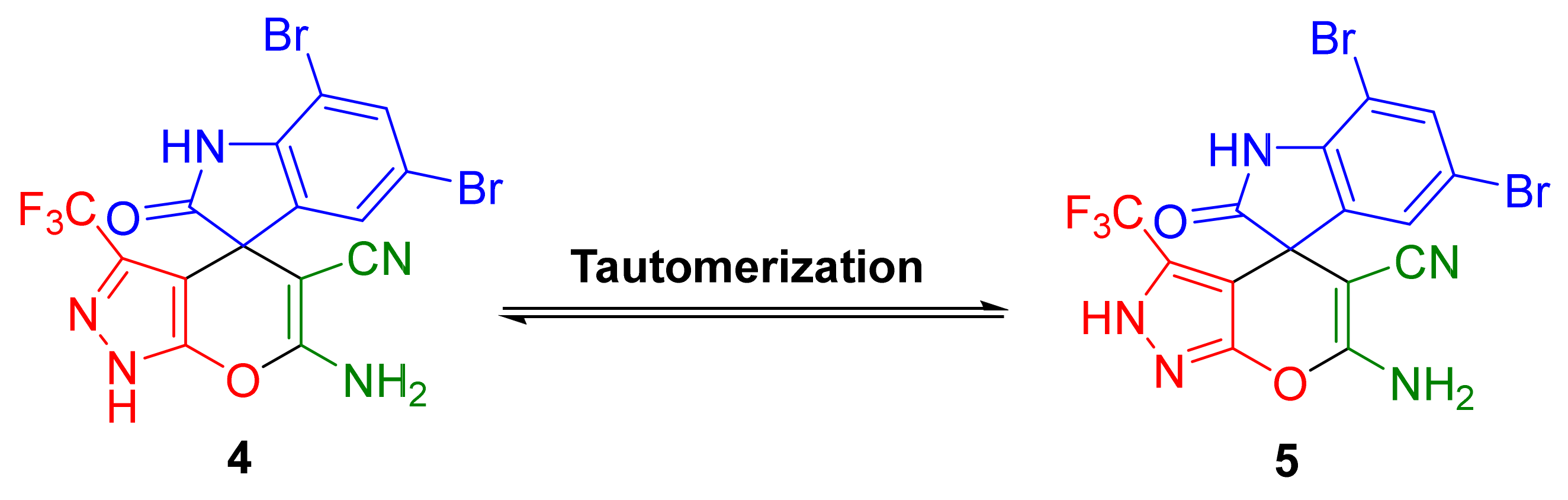
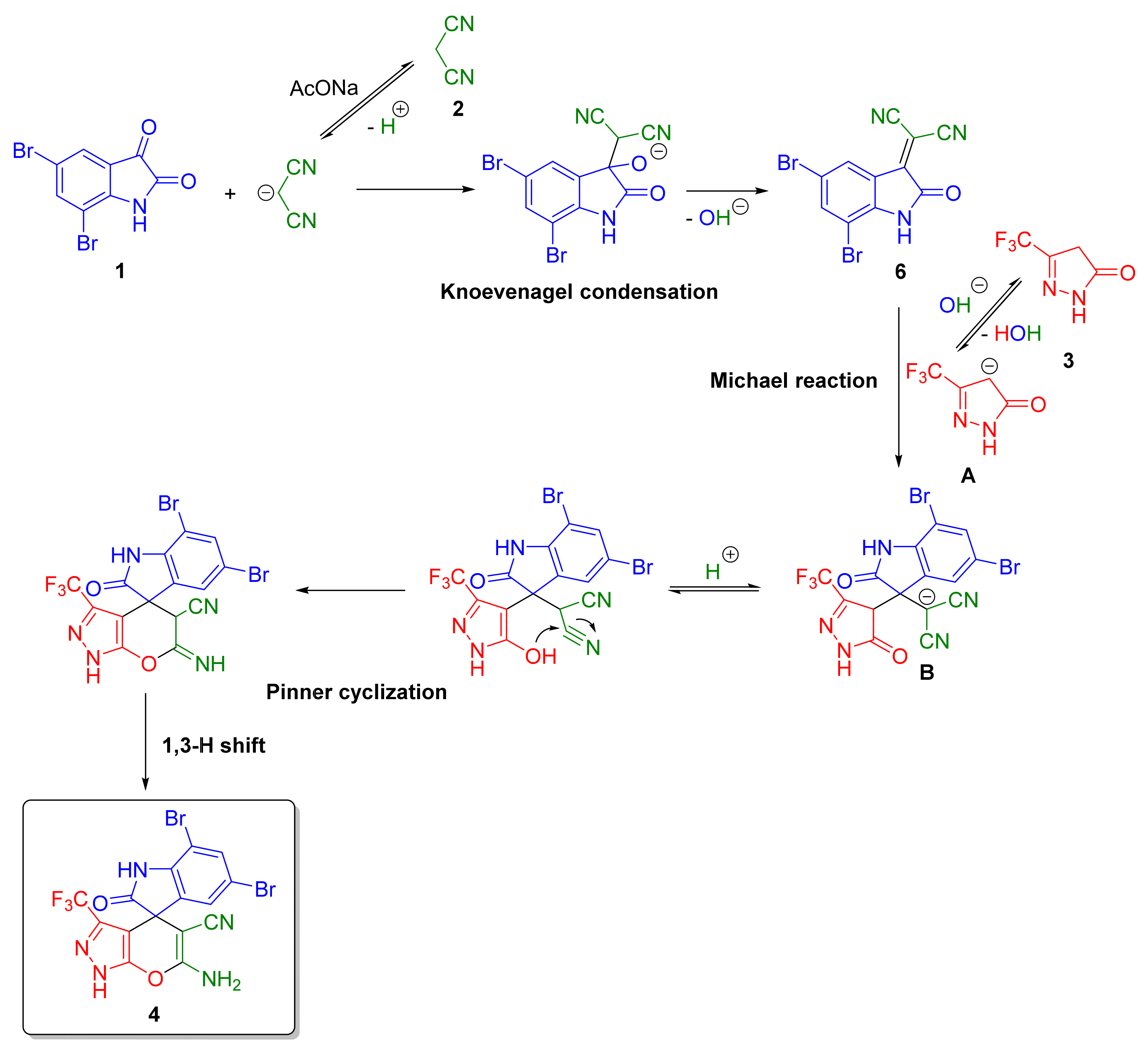
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Multicomponent Synthesis of 6′-Amino-5,7-dibromo-2-oxo-3′-(trifluoromethyl)-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, R.P.; Rajput, A.P. A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent. Today 2013, 5, 148–152. [Google Scholar] [CrossRef]
- Brahmachari, G. Green synthetic approaches for biologically relevant heterocycles: Advanced synthetic techniques—An overview. In Green Synthetic Approaches for Biologically Relevant Heterocycles (Second Edition), Volume 1: Advanced Synthetic Techniques; Brahmachari, G., Ed.; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 2021; Chapter 1; pp. 1–8. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Verma, M.; Tripathi, M.; Saxena, A.; Shanker, K. Antiinflammatory activity of novel indole derivatives. Eur. J. Med. Chem. 1994, 29, 941–946. [Google Scholar] [CrossRef]
- Praveen, C.; Ayyanar, A.; Perumal, P.T. Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4072–4077. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Yamamoto, Y.; Matsumura, Y.; Ohba, K.; Sakamaki, S.; Kimata, H.; Nakayama, K.; Kuriyama, C.; Matsushita, Y.; Ueta, K.; et al. Novel Indole-N-glucoside, TA-1887 as a sodium glucose Cotransporter 2 inhibitor for treatment of type 2 diabetes. ACS Med. Chem. Lett. 2014, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M. Synthesis under microwave irradiation of [1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011, 16, 8244–8256. [Google Scholar] [CrossRef] [PubMed]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef]
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers. 2016, 20, 299–344. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef]
- Sansinenea, E.; Martínez, E.F.; Ortiz, A. Organocatalytic Synthesis of Chiral Spirooxindoles with Quaternary Stereogenic Centers. Eur. J. Org. Chem. 2020, 2020, 5101–5118. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Korolev, V.A.; Egorov, M.P. Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold. Heterocycl. Commun. 2016, 22, 11–15. [Google Scholar] [CrossRef][Green Version]
- Elinson, M.N.; Ryzhkov, F.V.; Vereshchagin, A.N.; Zaimovskaya, T.A.; Egorov, M.P. Solvent-free multicomponent assembling of isatins, malononitrile, and dimedone: Fast and efficient way to functionalized spirooxindole system. Monatsh Chem. 2016, 147, 755–760. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Zaimovskaya, T.A.; Korolev, V.A.; Egorov, M.P. Multicomponent assembling of isatins, malononitrile and 4-hydroxy-6-methylpyridin-2(1H)-ones: One-pot efficient approach to privileged spiro[indoline-3,4′-pyrano[3,2-c]pyridine]-2,5′(6′H)-dione scaffold. Mendeleev Commun. 2016, 26, 399–401. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Ryzhkov, F.V.; Anisina, Y.E. Solvent-free and on-solvent multicomponent reaction of isatins, malononitrile, and bicyclic CH-acids: Fast and efficient way to medicinal privileged spirooxindole scaffold. Arkivoc 2018, 4, 276–285. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Wang, X.; Yang, W.; Qian, Q.; Zhang, M.; Song, L.; Deng, H.; Shao, M. A facile and convenient way to functionalized trifluoromethylated spirocyclic[indole-3,4-pyrano[2,3-c]pyrazole] derivatives. Tetrahedron Lett. 2013, 54, 4451–4455. [Google Scholar] [CrossRef]
- Schwertz, G.; Witschel, M.C.; Rottmann, M.; Leartsakulpanich, U.; Chitnumsub, P.; Jaruwat, A.; Amornwatcharapong, W.; Ittarat, W.; Schäfer, A.; Aponte, R.A.; et al. Potent Inhibitors of Plasmodial Serine Hydroxymethyltransferase (SHMT) Featuring a Spirocyclic Scaffold. ChemMedChem 2018, 13, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Santos, I.S.; Guerra, F.S.; Bernardino, L.F.; Fernandes, P.D.; Hamerski, L.; Silva, B.V. A Facile Synthesis of Novel Isatinspirooxazine Derivatives and Potential in vitro Anti-Proliferative Activity. J. Braz. Chem. Soc. 2019, 30, 198–209. [Google Scholar] [CrossRef]
- Bingi, C.; Emmadi, N.R.; Chennapuram, M.; Nanubolu, J.B.; Atmakur, K. A simple and catalyst free one pot access to the pyrazolone fused 2,8-dioxabicyclo[3.3.1]nonanes. RSC Adv. 2014, 4, 35009–35016. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Kalashnikova, V.M.; Elinson, M.N. 6′-Amino-5,7-dibromo-2-oxo-3′-(trifluoromethyl)-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile. Molbank 2022, 2022, M1309. https://doi.org/10.3390/M1309
Ryzhkova YE, Kalashnikova VM, Elinson MN. 6′-Amino-5,7-dibromo-2-oxo-3′-(trifluoromethyl)-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile. Molbank. 2022; 2022(1):M1309. https://doi.org/10.3390/M1309
Chicago/Turabian StyleRyzhkova, Yuliya E., Varvara M. Kalashnikova, and Michail N. Elinson. 2022. "6′-Amino-5,7-dibromo-2-oxo-3′-(trifluoromethyl)-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile" Molbank 2022, no. 1: M1309. https://doi.org/10.3390/M1309
APA StyleRyzhkova, Y. E., Kalashnikova, V. M., & Elinson, M. N. (2022). 6′-Amino-5,7-dibromo-2-oxo-3′-(trifluoromethyl)-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile. Molbank, 2022(1), M1309. https://doi.org/10.3390/M1309