Abstract
The natural product lupeol (1) was isolated from Bombax ceiba leaves, which were used as starting material in the semisynthetic approach. Three new derivatives (2a, 2b, and 3) were synthesized using oxidation and aldolization. Their chemical structures were elucidated by spectroscopic analyses (HRESIMS and NMR). Compounds 3 showed significant α-glucosidase inhibition with an IC50 value of 202 µM, whereas 2a and 2b were inactive.
1. Introduction
Diabetes mellitus (DM) causes high blood glucose after the consumption of a carbohydrate-enriched diet, leading to hyperglycemia. Uncontrolled diabetes is manifested by a very high rise in triglycerides and fatty acid levels [1]. Diverse antidiabetic drugs derived from synthetic compounds are of interest to chemists. However, these synthetic drugs come with several serious complications [1]. Due to the limitations associated with the use of existing synthetic antidiabetic drugs, the search for newer antidiabetic agents from natural sources continues. Lupeol is a pharmacologically active pentacyclic triterpenoid found in several medicinal plants worldwide [2]. It has several potential medicinal properties and is found in a variety of botanical sources [3]. Notably, lupeol has been reported to selectively target diseased and unhealthy human cells, while sparing normal and healthy cells [4]. Dozens of novel lupeol derivatives were synthesized and screened for their in vivo antihyperglycemic activity [5,6]. Most derivatives lowered the blood glucose levels, in a sucrose-challenged streptozotocin-induced diabetic rat (STZ-S) model [5]. To continue our ongoing search for highly efficient antidiabetic agents from derivatized lupeol [6,7], we herein describe the synthesis of lupeol derivatives 2, 2a, 2b, and 3 (Figure 1). The structures of all the obtained compounds were characterized by 1H, 13C NMR, and HRESIMS. All derivatives were evaluated for α-glucosidase inhibition.
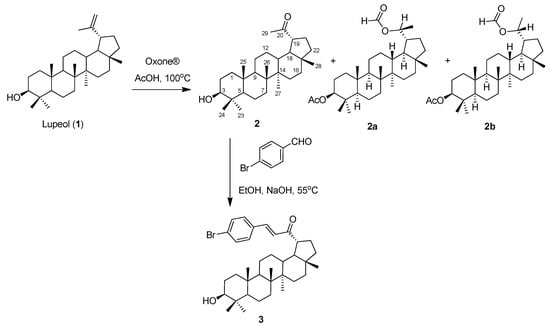
Figure 1.
Synthesis of 2, 2a, 2b, and 3 from lupeol (1).
2. Results and Discussion
2.1. Synthesis
Lupeol was isolated from the Vietnamese plant Bombax ceiba, following our previously reported procedure [8]. Lupeol was transformed to products 2, 2a, and 2b using oxidation with Oxone®, a potassium triple-salt (KHSO5·1/2KHSO4·1/2K2SO4) [6,9]. The conditions followed our previously reported method [6], with slight modifications. Both 2a and 2b had the same molecular formula as C32H52O4. Comparison of NMR data of 2a/2b and 1 indicated that oxidation occurred. The 1H NMR spectrum of 2a/2b showed differences with 1: the downfield methine at δH 8.11, two oxymethines at δH 5.26 and 4.48, and a doublet methyl at δH 1.22. These signals indicated that the isopropenyl group of 1 was transformed to a 2-formylethyl group at C-19. Moreover, the downfield signal of H-3 (δH 4.48) indicated that 3-OH was esterified by acetic acid. The 13C NMR spectrum of 2a/2b showed one carbonyl ester at δC 171.1, one formyl group at δC 163.7 and two oxygenated carbons at δC 81.1 and 72.7, supporting the previous findings. Interestingly, 2a and 2b are C-20 epimers. Corbett and co-workers [10,11] indicated the method to define the absolute configuration of C-20 of lupane-type triterpenes. Particularly, the (20S) and (20R) isomers exhibited differences in the chemical shifts of C-19, C-20, C-29, and C-30, especially C-30. According to Corbett et al., 2a, having C-30 at δC 20.1, would have a 20R configuration. On the other hand, 2b would have the 20S configuration due to the lower chemical shift of C-30 at δC 14.2.
Compound 2 was further aldolized with 4-bromobenzaldehyde to afford compound 3. Compound 3 had the same molecular formula as C36H51BrO2, determined by a protonated ion peak at m/z 595.3188 in HRESIMS. Comparison of 1D NMR data of 2 and 3 indicated obvious differences. The first difference is the presence of a 1,4-disubstituted benzenoid characterized by two ortho-coupled protons at δH 7.51 and 7.42, and a trans double bond at δH 6.75 and 7.46. This was confirmed by the disappearance of a methyl ketone group at δH 2.15 (CH3-29). This finding indicated that the aldolization occurred exclusively at C-29. The second difference was in the 13C NMR spectrum. This spectrum showed the presence of seven aromatic carbons at δC 141.0 (C-1), 133.9 (C-5′), 132.3 (C-2′), 129.8 (C-3′,7′), and 126.9 (C-4′, 6′), supporting the reaction at C-29.
2.2. α-Glucosidase Inhibition of 2a, 2b, and 3
Compounds 2a, 2b, and 3 were evaluated for α-glucosidase inhibition. Only compound 3 exhibited moderate α-glucosidase inhibition with an IC50 value of 202 µM, compared with an acarbose-positive control (IC50 360 μM). Other compounds were inactive.
3. Materials and Methods
3.1. Materials
Reagents and solvents were obtained from commercial suppliers and were used without further purification. Column chromatography was carried out using Merck Kieselgel 60 silica gel (particle size: 32–63 Å). Analytical TLC was performed using Merck precoated silica gel 60 F-254 sheets.
NMR spectroscopic data were acquired on Bruker Avance III apparatus at 500 MHz for 1H NMR and 125 MHz for 13C NMR. HRESIMS spectra were recorded on a Bruker MICROTOF-Q 10187.
Extraction and Isolation. The air-dried Bombax ceiba leaves (4 kg) were ground into powder and exhaustively extracted at room temperature with MeOH (2 × 10 L). The filtered solution was evaporated under reduced pressure to afford a residue (473.4 g). This crude extract was subsequently partitioned using solvents of n-hexane and EtOAc to yield n-hexane (40 g) and EtOAc (88 g) extracts. The n-hexane extract was fractionated by silica gel column chromatography (CC), eluted with n-hexane–EtOAc (isocratic, 10:1, v/v), to produce five fractions (H1-H5). Fraction H2 (15 g) was rechromatographed by silica gel CC using n-hexane–CHCl3 (isocratic, 12:1, v/v) as eluent to afford lupeol (1) (1.5 g).
3.2. Synthesis Procedure
Synthesis of 2, 2a, and 2b: Lupeol (1, 200 mg, 0.469 mmol) was oxidized with Oxone® (951 mg, 1.548 mmol) in acetic acid (40 mL) at 100 °C for 3 h. The mixture was stirred and continuously monitored by TLC. The mixture was extracted with EtOAc–water (1:1) to gain the organic layer. This solution was evaporated to afford a residue. Then, the residue was purified by silica gel CC to give compounds 2, 2a, and 2b.
Compound 2. Isolated yield: 74.6 mg (37%), white solid. 1H and 13C NMR data were consistent with those reported previously [6].
(3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bS)-1-((S)-1-(formyloxy)ethyl)-3a,5a,5b,8,8,11a-hexamethylicosahydro-1H-cyclopenta[a]chrysen-9-yl acetate (2a). Isolated yield: 9.4 mg (4%), white solid. 1H NMR (500 MHz, CDCl3, δ, ppm): 2.05 (3H, s, CH3-2′), 8.00 (1H, s, OCHO-29), 5.33 (1H, m, H-20), 4.48 (1H, dd, J = 10.5, 6.0 Hz, H-3), 2.13 (1H, m, H-3), 1.18 (3H, d, J = 6.5 Hz, CH3-30), 1.03 (3H, s, CH3-26), 0.90 (3H, s, CH3-27), 0.87 (3H, s, CH3-25), 0.85 (3H, s, CH3-23), 0.84 (3H, s, CH3-24), 0.79 (1H, d, J = 9.5 Hz, H-5), 0.76 (3H, s, CH3-28). 13C NMR (125 MHz, CDCl3, δ, ppm): 171.2 (C-6′), 21.5 (C2′), 161.6 (C-29), 81.1 (C-3), 73.4 (C-20), 55.5 (C-5), 50.2 (C-9), 48.8 (C-18), 43.5 (C-17), 43.0 (C-14), 42.6 (C-19), 41.0 (C-8), 40.5 (C-22), 38.5 (C-4), 38.0 (C-1), 37.3 (C-13), 37.2 (C-10), 35.5 (C-16), 34.4 (C-7), 29.9 (C-21), 28.1 (C-23), 27.3 (C-15), 27.1 (C-2), 23.8 (C-12), 21.0 (C-11), 18.4 (C-6), 18.1 (C-28), 16.7 (C-25), 16.4 (C-26), 16.1 (C-24), 14.4 (C-27), 14.2 (C-30). HRESIMS calcd C32H52NaO4 ([M+Na]+): 523.3732, found: 523.3763.
(3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bS)-1-((R)-1-(formyloxy)ethyl)-3a,5a,5b,8,8,11a-hexamethylicosahydro-1H-cyclopenta[a]chrysen-9-yl acetate (2b). Yield: 9.4 mg (5%), white solid. 1H NMR (500 MHz, CDCl3, δ, ppm): 2.04 (3H, s, H-2′), 8.11 (1H, s, OCHO-29), 5.26 (1H, m, H-20), 4.48 (1H, dd, J = 11.5, 5.5 Hz, H-3), 2.31 (1H, m, H-19), 1.22 (3H, d, J = 6.5 Hz, CH3-30), 1.03 (3H, s, CH3-26), 0.86 (3H, s, CH3-25), 0.85 (3H, s, CH3-27), 0.85 (3H, s, CH3-23), 0.84 (3H, s, CH3-24), 0.77 (1H, d, J = 2.0 Hz, H-5), 0.75 (3H, s, CH3-28). 13C NMR (125 MHz, CDCl3, δ, ppm): 171.1 (C-6′), 21.5 (C2′), 163.7 (C-29), 81.1 (C-3), 72.7 (C-20), 55.5 (C-5), 50.0 (C-9), 47.1 (C-18), 44.4 (C-19), 43.2 (C-17), 43.0 (C-14), 41.0 (C-8), 40.1 (C-22), 38.5 (C-4), 38.0 (C-1), 37.5 (C-13), 37.3 (C-10), 35.3 (C-16), 34.4 (C-7), 29.9 (C-21), 28.1 (C-23), 27.4 (C-15), 26.9 (C-2), 23.9 (C-12), 21.0 (C-11), 20.1 (C-30), 18.4 (C-6), 18.1 (C-28), 16.7 (C-25), 16.3 (C-26), 16.1 (C-24), 14.4 (C-27). HRESIMS calcd C32H52NaO4 ([M+Na+H2O]+): 541.3870, found: 541.3869.
Synthesis of 3: Compound 2 (70 mg, 0.163 mmol) together with NaOH (35 mg, 0.875 mmol) in ethanol (7 mL) was stirred at 55 °C for 15 min. Then, 4–bromobenzaldehyde (64.35 mg, 0.35 mmol) was added to the mixture. The reaction was performed at 55 °C for 2 h. The mixture was extracted with EtOAc–water (1:1, v/v) to gain the organic layer. This solution was applied to silica gel CC using the gradient system of n-hexane–EtOAc (10:1, v/v) to obtain compound 3. Isolated yield: 68 mg (48%), white solid.
(E)-3-(4-bromophenyl)-1-((1R,3aR,5aR,5bR,9S,11aR)-9-hydroxy-3a,5a,5b,8,8,11a-hexamethylicosahydro-1H-cyclopenta[a]chrysen-1-yl)prop-2-en-1-one (3): 1H NMR (500 MHz, CDCl3, δ, ppm): 7.51 (2H, d, J = 8.5 Hz, H-3′,7′), 7.46 (1H, d, J = 16.0 Hz, H-6′), 7.42 (2H, d, J = 8.5 Hz, H-4′,6′), 6.75 (1H, d, J = 16.0 Hz, H-29), 3.19 (1H, dd, J = 11.2, 4.8 Hz, H-3), 2.87 (1H, td, J = 11.5, 6.0 Hz, H-19), 1.02 (3H, s, CH3-26), 0.98 (3H, s, CH3-27), 0.96 (3H, s, CH3-23), 0.84 (3H, s, CH3-24), 0.80 (3H, s, CH3-25), 0.75 (3H, s, CH3-28). 13C NMR (125 MHz, CDCl3, δ, ppm): 204.1 (C-20), 141.0 (C-6′), 133.9 (C-5′), 132.3 (C-3′,7′), 129.8 (C-4′,6 ‘), 126.9 (C-29), 124.7 (C-2′), 79.0 (C-3), 55.4 (C-5), 50.4 (C-9), 50.1 (C-18), 43.3 (C-17), 42.9 (C-14), 40.9 (C-8), 40.3 (C-22), 39.0 (C-4), 38.8 (C-1), 37.3 (C-10), 35.2 (C-16), 34.3 (C-7), 28.7 (C-15), 28.1 (C-23), 27.9 (C-2), 27.5 (C-12), 21.1 (C-11), 18.4 (C-6), 18.3 (C-28), 16.2 (C-26), 16.0 (C-25), 15.5 (C-24), 14.6 (C-27). HRESIMS calcd C36H52BrO2 ([M−H]−): 595.3151, found: 595.3188.
3.3. α-Glucosidase Inhibitory Assay
The α-glucosidase (0.2 U/mL) and substrate (5.0 mM p-nitrophenyl-α-D-glucopyranoside) were dissolved in 100 mM pH 6.9 sodium phosphate buffer [12]. The inhibitor (50 µL) was preincubated with α-glucosidase; then, the substrate (40 µL) was added to the reaction mixture. The enzymatic reaction was carried out at 37 °C for 20 min and stopped by the addition of 0.2 M Na2CO3 (130 μL). Enzymatic activity was quantified by measuring absorbance at 405 nm. All samples were analyzed in triplicate at five different concentrations around the IC50 values, and the mean values were retained. The inhibition percentage (%) was calculated as follows: Inhibition (%) = [1 − (Asample/Acontrol)] × 100.
4. Conclusions
Three new derivatives, 2a, 2b, and 3, from the natural product lupeol have been synthesized via oxidation and aldolization routes and evaluated for their α-glucosidase inhibition. Synthetic compound 3 showed much stronger α-glucosidase inhibitory activity (IC50 202 μM) than acarbose (IC50 360 μM). Synthetic products 2a and 2b, which lacked the 3-OH group, exhibited lower activity than 3 toward α-glucosidase. This result confirmed that this substituted group might be involved in α-glucosidase inhibition.
Supplementary Materials
The following are available online. Copies of HRESIMS and NMR spectra for compound 2a, 2b, and 3.
Author Contributions
Conceptualization, T.-H.D. and J.S. methodology, T.-H.D. and J.S.; formal analysis, H.-T.-T.L., T.-H.D. and J.S.; investigation, T.-H.-T.N., H.-T.-T.L., Q.-C.C., T.-H.D. and Q.-T.-P.T.; data curation, N.-K.-T.P. and N.-H.N.; writing—original draft preparation, T.-H.D. and J.S.; writing—review and editing, T.-H.D. and J.S.; supervision, T.-H.D.; project administration, T.-H.D. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by The Youth Incubator for Science and Technology Programme, managed by the Youth Development Science and Technology Center—Ho Chi Minh Communist Youth Union and Department of Science and Technology of Ho Chi Minh City (38/2020/HĐ-KHCNT-VƯ). This work was also supported by Thammasat University Research Unit in Natural Products Chemistry and Bioactivities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the compounds presented in this study are available in the Supplementary Materials of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tabish, S.A. Is diabetes becoming the biggest epidemic of the twenty-first century? Int. J. Health Sci. 2007, 1, 3–8. [Google Scholar]
- Gallo, M.B.C.; Sarachine, M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009, 3, 46–66. [Google Scholar]
- Starks, C.M.; Williams, R.B.; Norman, V.L.; Lawrence, J.A.; Goering, M.G.; O’Neil-Johnson, M.; Hu, J.F.; Rice, S.M.; Eldridge, G.R. Abronione, a rotenoid from the desert annual Abronia villosa. Phytochem. Lett. 2011, 4, 72–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Papi Reddy, K.; Singh, A.B.; Puri, A.; Srivastava, A.K.; Narender, T. Synthesis of novel triterpenoid (lupeol) derivatives and their in vivo antihyperglycemic and antidyslipidemic activity. Bioorg. Med. Chem. Lett. 2009, 19, 4463–4466. [Google Scholar] [CrossRef]
- Phan, H.V.T.; Duong, T.H.; Pham, D.D.; Pham, H.A.; Nguyen, V.K.; Nguyen, T.P.; Nguyen, H.H.; Nguyen, N.H.; Sam-ang, P.; Phontree, K.; et al. Design and synthesis of new lupeol derivatives and their α-glucosidase inhibitory and cytotoxic activities. Nat. Prod. Res. 2020, Article in press. [Google Scholar] [CrossRef] [PubMed]
- Sichaem, J.; Aree, T.; Lugsanangarm, K.; Tip-Pyang, S. Identification of highly potent α-glucosidase inhibitory and antioxidant constituents from Zizyphus rugosa bark: Enzyme kinetic and molecular docking studies with active metabolites. Pharm. Biol. 2017, 55, 1436–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sichaem, J.; Inthanon, K.; Funnimid, N.; Phontree, K.; Phan, H.V.T.; Tran, T.M.D.; Niamnont, N.; Srikittiwanna, K.; Sedlak, S.; Duong, T.H. Chemical constituents of the stem bark of Bombax ceiba. Chem. Nat. Compd. 2020, 56, 909–911. [Google Scholar] [CrossRef]
- Uyanik, M.; Akakura, M.; Ishihara, K. 2-Iodoxybenzenesulfonic acid as an extremely active catalyst for the selective oxidation of alcohols to aldehydes, ketones, carboxylic acids, and enones with Oxone. J. Am. Chem. Soc. 2009, 131, 251–262. [Google Scholar] [CrossRef]
- Corbett, R.E.; Cong, A.N.T.; Wilkins, A.L.; Thomson, R.A. Lichens and Fungi. Part 17. The synthesis and absolute configuration at C-20 of the (R)- and (S)-epimers of some 29-substituted lupane derivatives and of some 30-norlupan-20-ol derivatives and the crystal structure of (20R)-3β-acetoxylupan-29-ol. J. Chem. Soc. Perkin Trans. 1985, 17, 2051–2056. [Google Scholar] [CrossRef]
- Corbett, R.E.; Cong, A.N.T.; Holland, P.T.; Wilkins, A.L. Lichens and Fungi. XVIII. Extractives from Pseudocyphellaria rubella. Aust. J. Chem. 1987, 40, 461–468. [Google Scholar] [CrossRef]
- Dao, T.B.N.; Nguyen, T.M.T.; Nguyen, V.Q.; Tran, T.M.D.; Tran, N.M.A.; Nguyen, C.H.; Nguyen, T.H.T.; Nguyen, H.H.; Sichaem, J.; Tran, C.L.; et al. Flavones from Combretum quadrangulare growing in Vietnam and their alpha-glucosidase inhibitory activity. Molecules 2021, 26, 2531. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).