Abstract
The molecular structure of bis(pyrazol-1-yl)methane-4,4′-dicarboxylic acid (H2bpmdc) was determined by single crystal X-Ray diffraction analysis. The compound crystallizes in a monoclinic crystal system; the unit cell contains four formula units. The molecules of H2bpmdc are linked into zig-zag chains by intermolecular carboxyl–carboxyl hydrogen bonds. Other types of supramolecular interactions, namely, CH···N and CH···O short contacts, CH–π interactions and carbonyl–carbonyl interactions were detected in the crystal structure.
1. Introduction
Dicarboxylic acids are important supramolecular synthons for metal–organic frameworks [1,2], hydrogen-bonded networks [3], organogels [4], deep eutectic solvents [5] and other applications [6]. Pyrazole-4-carboxylic acid and its derivatives have demonstrated potent biological activity [7,8]; they were also used for construction of highly porous metal–organic frameworks [9]. Dicarboxylic acids derived from bis(pyrazol-1-yl)methane have been less explored, but were successfully employed as building blocks for metal–organic frameworks with luminescent properties [10,11], gas separation capability [12], and single metal site catalysts [13,14].
Recently, we have developed a universal approach for the synthesis of a new series of bis(pyrazol-1-yl)alkane-4,4′-dicarboxylic acids starting from the commercially available pyrazole-4-carboxylic acid [15]. Taking into account the potential of these compounds as supramolecular building block, biologically active substances, monomers for polyesters and polyamides, we have studied the crystal structure and supramolecular analysis of N-heterocyclic compound titled bis(pyrazol-1-yl)methane-4,4′-dicarboxylic acid (H2bpmdc). This dicarboxylic acid was synthesized recently in our group and was characterized by NMR and IR spectroscopy, thermal and elemental analyses [15]; however, its crystal structure determination has not been performed yet.
2. Results and Discussion
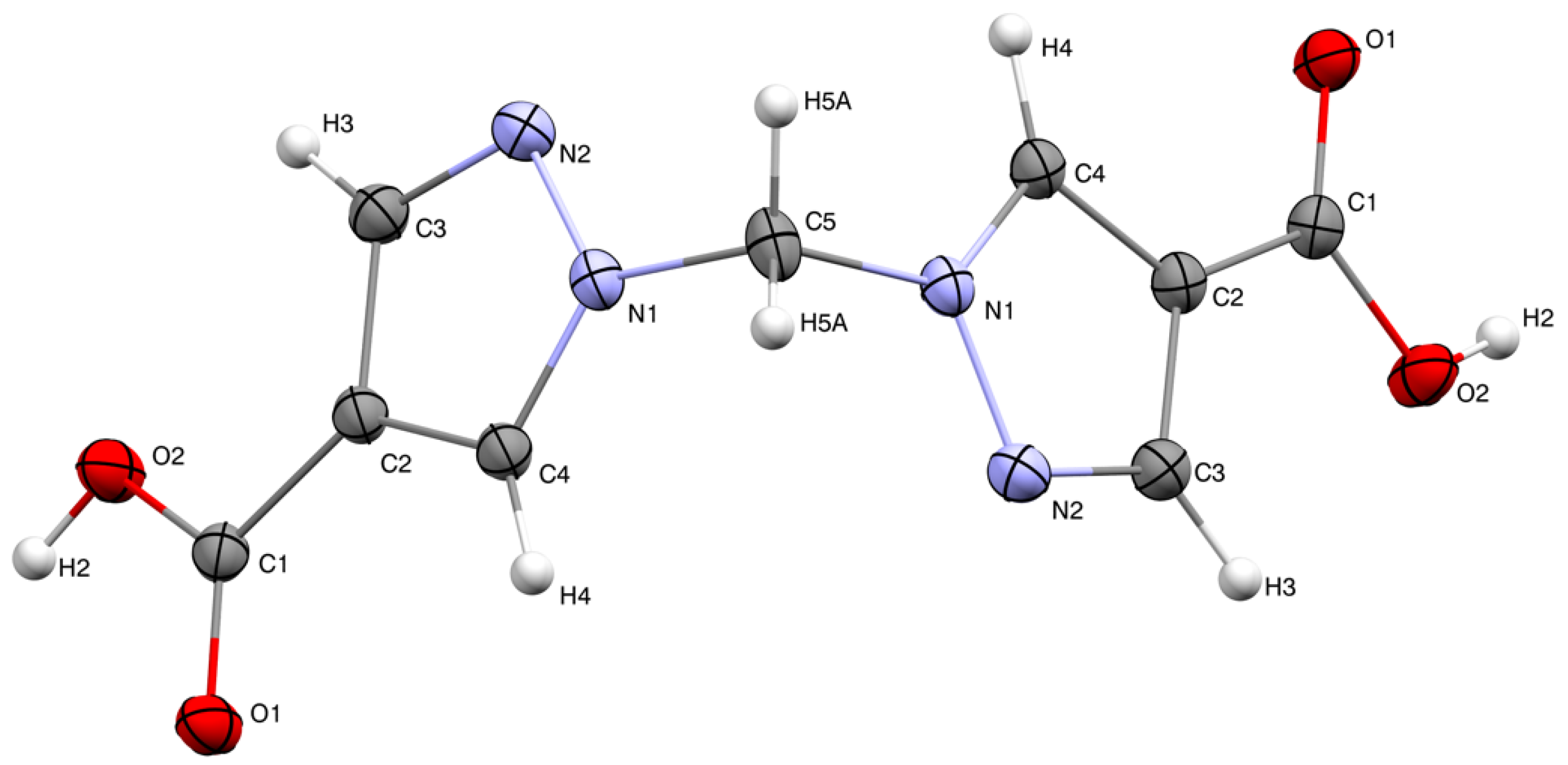
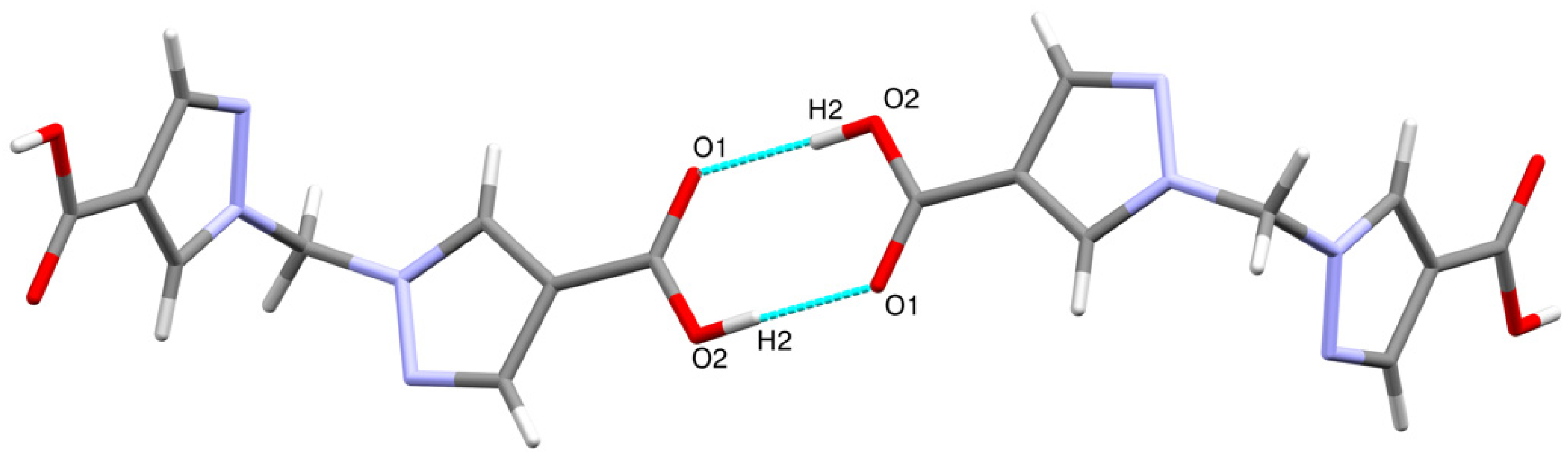
The molecular structure of H2bpmdc is shown in Figure 1. The compound crystallizes in a monoclinic crystal system, space group C2/c. The asymmetric unit consists of a half of the molecule and the unit cell contains four formula units. The angle between the planes of two pyrazole rings is 81.24(8)°, while the angle N1-C5-N1 is closer to tetrahedral (111.3(8)°). The neighboring molecules are involved in intermolecular hydrogen bonding via the carboxylic groups (Figure 2), the D-H distance, d(O2-H2) = 0.87(1) Å, A-H distance d(O1-H2) = 1.79(1) Å and D-H-A angle (O2-H2-O1) is 177(1)°. The interatomic distance d(O1-O2) = 2.655(1) Å is in the range typical for a carboxyl–carboxyl cyclic dimer motif [16].
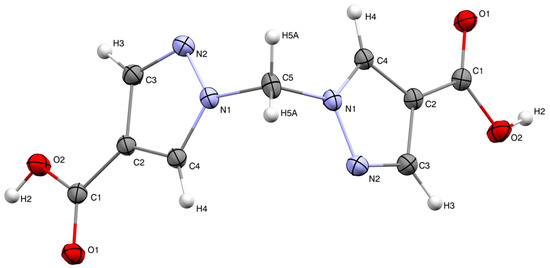
Figure 1.
Molecular structure of H2bpmdc; thermal ellipsoids are drawn at the 50% probability level.
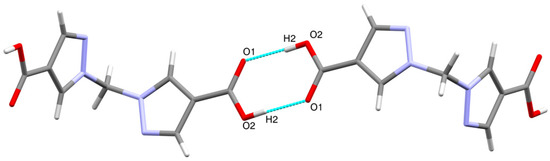
Figure 2.
Hydrogen-bonded carboxyl–carboxyl cyclic dimers between H2bpmdc molecules.
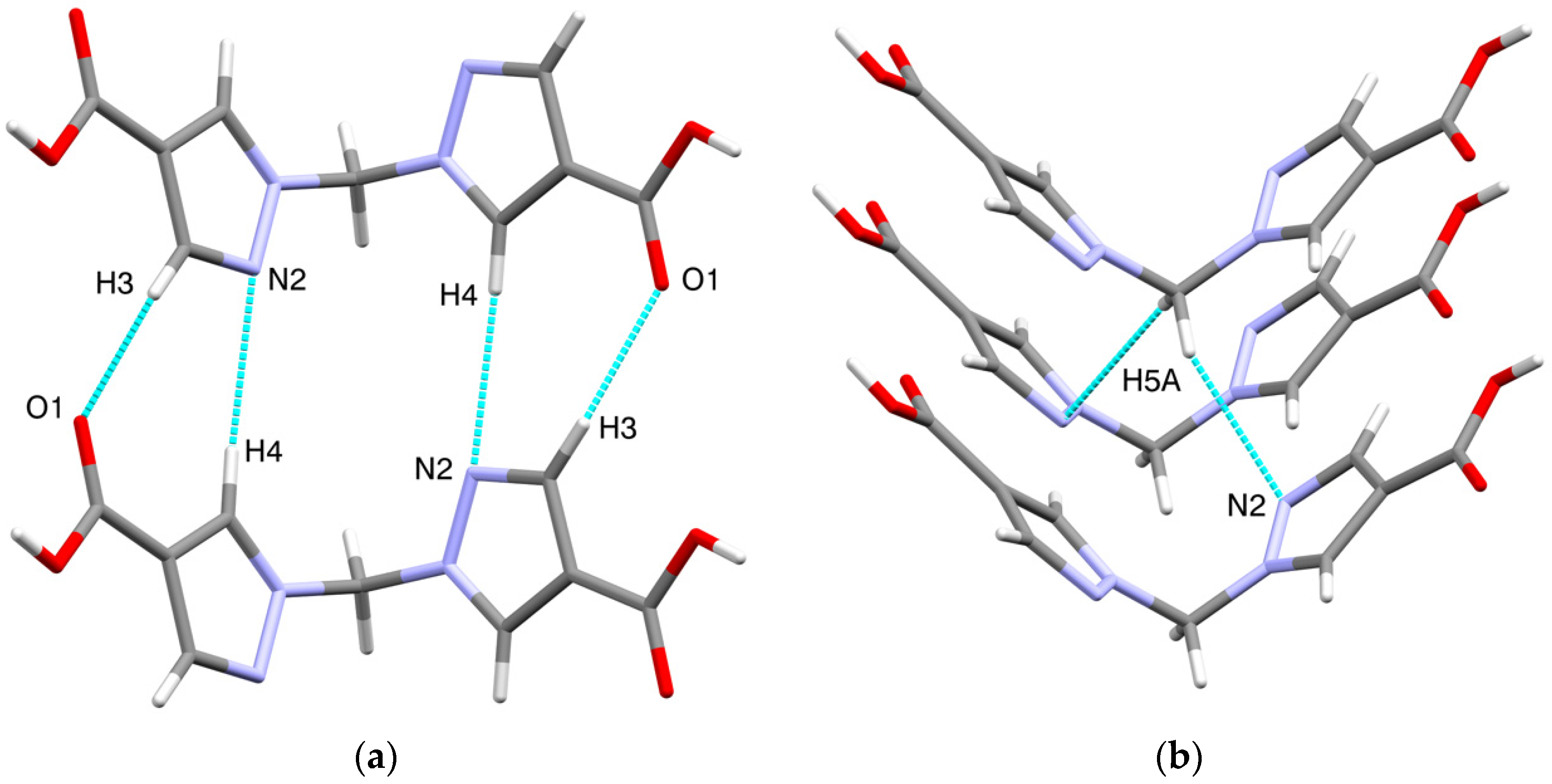
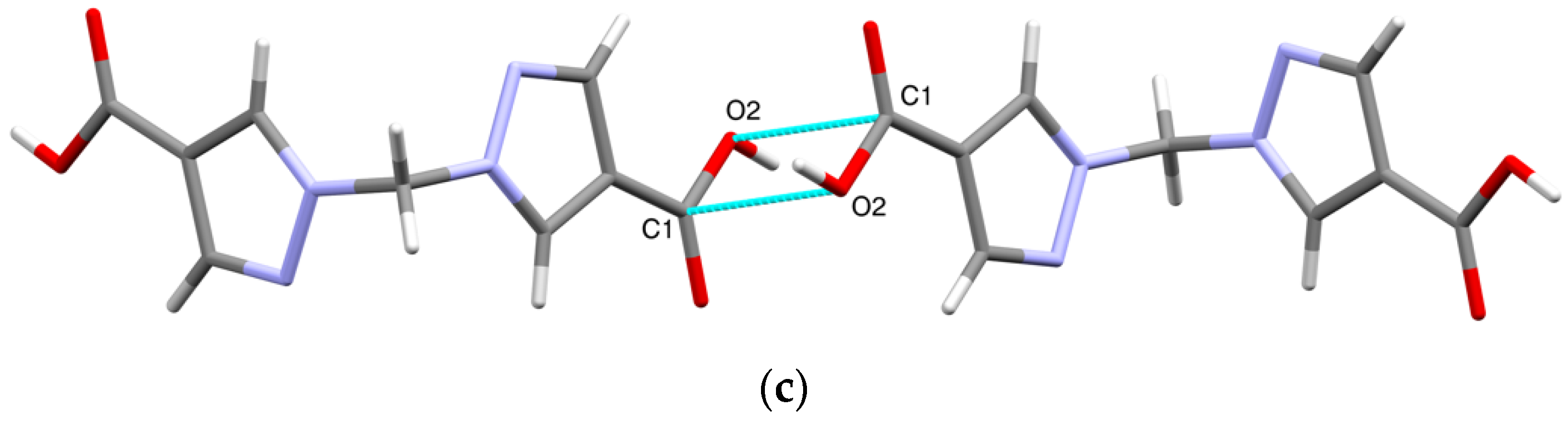
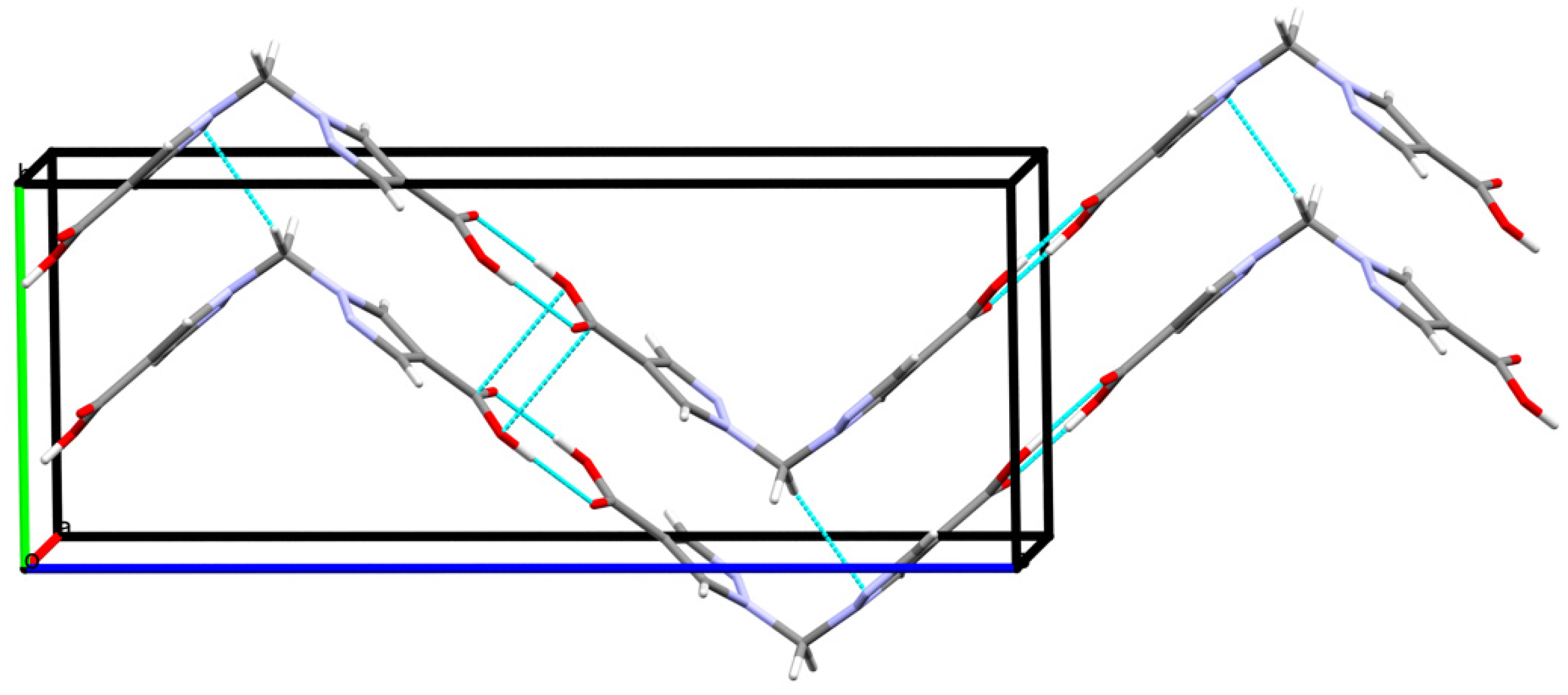
Other types of intermolecular interactions include CH···N and CH···O short contacts (Figure 3a) with the distances of 2.62(1) and 2.88(1) Å, correspondingly, CH–π interactions between CH2 groups and pyrazole rings (d(N2-H5A) = 2.716(7) Å, Figure 3b) and carbonyl–carbonyl interactions, d(C1-O2) = 3.170(1) A (Figure 3c). Hydrogen bonds link the H2bpmdc molecules into zig-zag chains oriented along the crystallographic axis c, while the above-mentioned interactions join the chains into supramolecular stacks along the axis b (Figure 4). Selected geometric parameters of H2bpmdc are listed in Table 1.
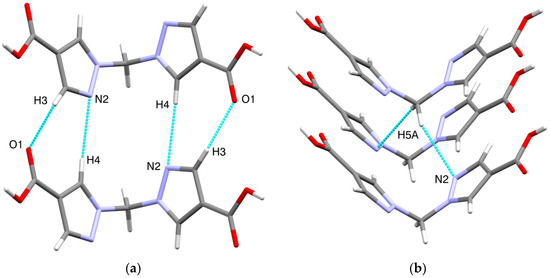
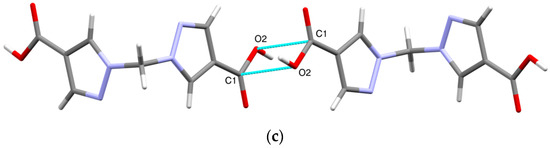
Figure 3.
Intermolecular interactions in the crystal structure of H2bpmdc: (a) short contacts CH···N and CH···O; (b) CH–π interactions; (c) carbonyl–carbonyl interactions.
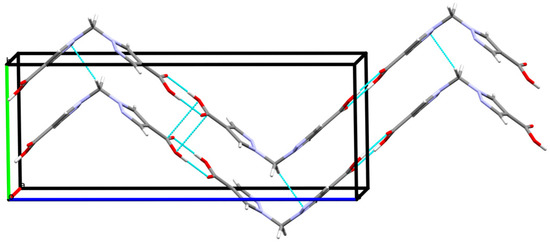
Figure 4.
Hydrogen-bonded chains of H2bpmdc molecules.

Table 1.
Selected geometric parameters of the molecular structure of H2bpmdc.
3. Materials and Methods
Bis(pyrazol-1-yl)methane-4,4′-dicarboxylic acid (H2bpmdc) was synthesized as described previously [15] and recrystallized from water to give single crystals suitable for X-ray crystal structure determination.
Single crystal XRD data for H2bpmdc were collected with a Bruker D8 Venture diffractometer with a CMOS PHOTON III detector and IµS 3.0 source (mirror optics, λ(MoKα) = 0.71073Å). The φ- and ω-scan techniques were employed to measure intensities. The crystal structure was solved using the SHELXT [17] and was refined using SHELXL [18] programs with OLEX2 GUI [19]. Atomic displacement parameters for non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed geometrically and treated as a mixture of independent and constrained refinement.
Crystal Data for C9H8N4O4 (M = 236.19 g/mol): monoclinic, space group C2/c, a = 5.7619(7), b = 8.0578(11), c = 20.806(2) Å, β = 90.370(4)°, V = 966.0(2) Å3, Z = 4, T = 150(2) K, μ(MoKα) = 0.12 mm−1, Dcalc = 1.624 g/cm3, 9866 reflections measured (3.92° ≤ 2Θ ≤ 33.16°), 1406 unique (Rint = 0.045, Rsigma = 0.039). The final R1 was 0.0393 (I > 2σ(I)) and wR2 was 0.110 (all data).
Full crystallographic information (as CIF file) along with CheckCIF report are given in the supplementary materials.
Supplementary Materials
The following are available online. Crystallographic information file (CIF) and CheckCIF report for compound H2bpmdc.
Author Contributions
Conceptualization, A.S.P.; methodology, A.S.P.; investigation, E.A.P., D.I.P., N.P.B.; writing—original draft preparation, D.I.P., E.A.P.; writing—review and editing, A.S.P.; supervision, A.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Ministry of Science and Higher Education of the Russian Federation, project number 121031700321-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
CCDC 2118484 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at http://www.ccdc.cam.ac.uk/data_request/cif (last accessed 18 November 2021).
Acknowledgments
The authors thank D.A. Piryazev for providing the data collected in XRD Facility of NIIC SB RAS.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hawes, C.S. Coordination sphere hydrogen bonding as a structural element in metal–organic Frameworks. Dalton Trans. 2021, 50, 6034–6049. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Shi, Z.-Q.; Ji, N.-N.; Guo, K.-M.; Li, G. Crystalline hydrogen-bonded supramolecular frameworks (HSFs) as new class of proton conductive materials. Appl. Surf. Sci. 2020, 504, 144484. [Google Scholar] [CrossRef]
- Liao, L.; Zhong, X.; Jia, X.; Liao, C.; Zhong, J.; Ding, S.; Chen, C.; Hong, S.; Luo, X. Supramolecular organogels fabricated with dicarboxylic acids and primary alkyl amines: Controllable self-assembled structures. RSC Adv. 2020, 10, 29129–29138. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional supramolecular systems: Design and applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Röhm, S.; Schröder, M.; Dwyer, J.E.; Widdowson, C.S.; Chaikuad, A.; Berger, B.-T.; Joerger, A.C.; Krämer, A.; Harbig, J.; Dauch, D.; et al. Selective targeting of the αC and DFG-out pocket in p38 MAPK. Eur. J. Med. Chem. 2020, 208, 112721. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.M.; Junior, H.C.S.; Costa, S.C.; Cardoso, C.M.; Cruz, A.G.B.; Santos, C.E.R.; Candela, D.R.S.; Soriano, S.; Marques, M.M.; Ferreira, G.B.; et al. Mononuclear coordination compounds containing a pyrazole-based ligand: Syntheses, magnetism and acetylcholinesterase inhibition assays. J. Mol. Struct. 2020, 1205, 127564. [Google Scholar] [CrossRef]
- Liu, Q.; Song, Y.; Ma, Y.; Zhou, Y.; Cong, H.; Wang, C.; Wu, J.; Hu, G.; O’Keeffe, M.; Deng, H. Mesoporous Cages in Chemically Robust MOFs Created by a Large Number of Vertices with Reduced Connectivity. J. Am. Chem. Soc. 2019, 141, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Wang, Q.; Bao, J.; Wu, Y.; Sun, L.; Yang, B.; Liu, Q. Synthesis and structural diversity of d 10 metal coordination polymers constructed from new semi-rigid bis(3-methyl-1H-pyrazole-4-carboxylic acid)alkane ligands. New J. Chem. 2017, 41, 5151–5160. [Google Scholar] [CrossRef]
- Radi, S.; El-Massaoudi, M.; Benaissa, H.; Adarsh, N.N.; Ferbinteanu, M.; Devlin, E.; Sanakis, Y.; Garcia, Y. Crystal engineering of a series of complexes and coordination polymers based on pyrazole-carboxylic acid ligands. New J. Chem. 2017, 41, 8232–8241. [Google Scholar] [CrossRef]
- Kivi, C.E.; Gelfand, B.S.; Dureckova, H.; Ho, H.T.K.; Ma, C.; Shimizu, G.K.H.; Woo, T.K.; Song, D. 3D porous metal–organic framework for selective adsorption of methane over dinitrogen under ambient pressure. Chem. Commun. 2018, 54, 14104–14107. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.M.; Burgun, A.; Coghlan, C.J.; Lee, R.; Coote, M.L.; Doonan, C.J.; Sumby, C.J. Capturing snapshots of post-synthetic metallation chemistry in metal–organic frameworks. Nat. Chem. 2014, 6, 906. [Google Scholar] [CrossRef] [PubMed]
- Burgun, A.; Coghlan, C.J.; Huang, D.M.; Chen, W.; Horike, S.; Kitagawa, S.; Alvino, J.F.; Metha, G.F.; Sumby, C.J.; Doonan, C.J. Mapping-Out Catalytic Processes in a Metal-Organic Framework with Single-Crystal X-ray Crystallography. Angew. Chemie Int. Ed. 2017, 56, 8412–8416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burlutskiy, N.P.; Potapov, A.S. Approaches to the Synthesis of Dicarboxylic Derivatives of Bis(pyrazol-1-yl)alkanes. Molecules 2021, 26, 413. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, L.; Auffinger, P. A comprehensive classification and nomenclature of carboxyl-carboxyl(ate) supramolecular motifs and related catemers: Implications for biomolecular systems. Acta Crystallogr. Sect. B 2015, 71, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).