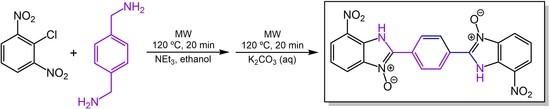
2,2′-(1,4-Phenylene)bis(7-nitro-1H-benzimidazole 3-oxide)
Abstract
:1. Introduction
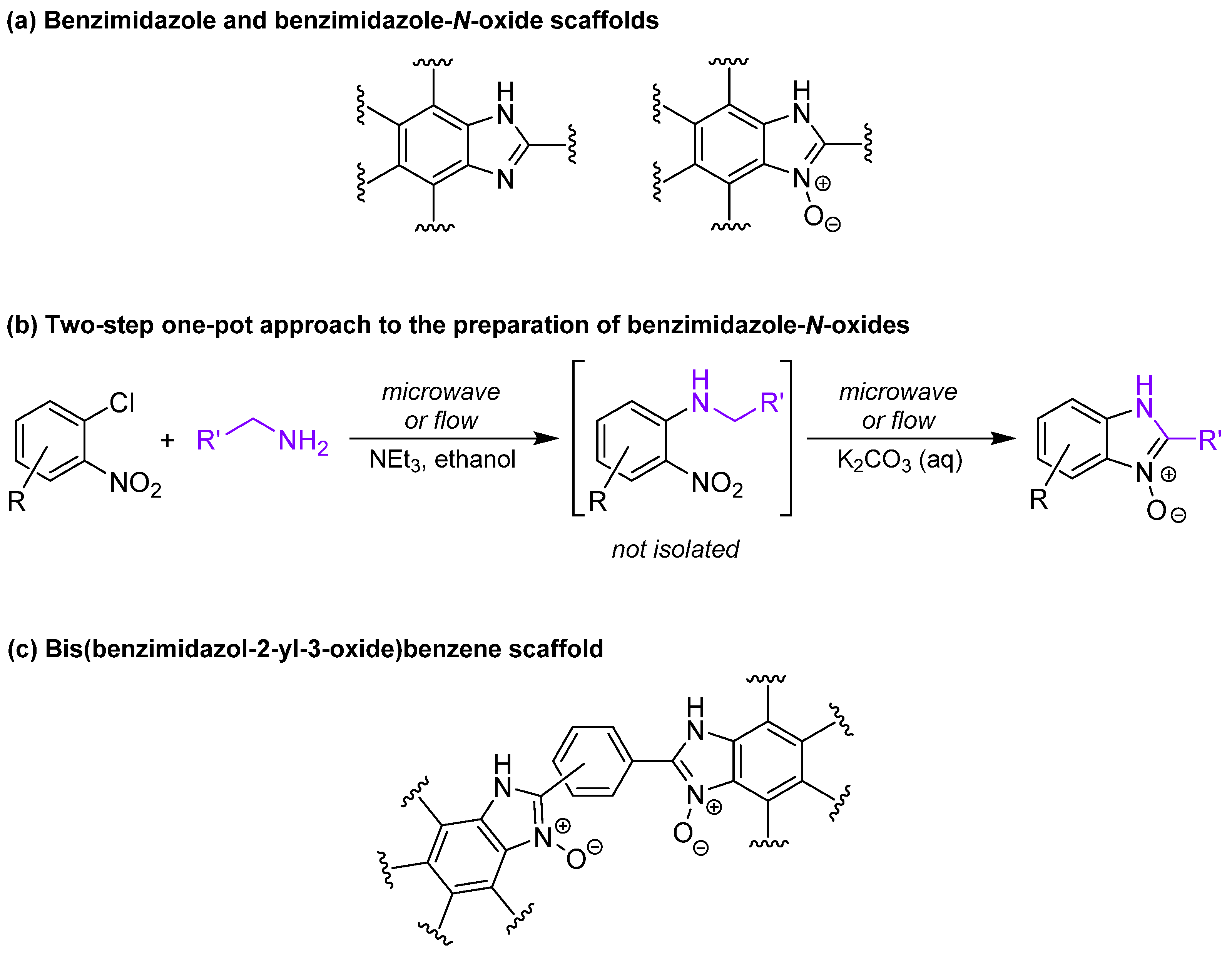
2. Results
3. Materials and Methods
3.1. General
3.2. Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meanwell, N.A.; Lolli, M.L. (Eds.) Applications of Heterocycles in the Design of Drugs and Agricultural Products; Elsevier: Oxford, UK, 2021. [Google Scholar]
- Li, J. (Ed.) Heterocyclic Chemistry in Drug Discovery; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.-H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef] [PubMed]
- Mlostoń, G. Heterocycles in Materials Chemistry. Chem. Heterocycl. Compd. 2017, 53, 1. [Google Scholar] [CrossRef] [Green Version]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological Significance of Heterocyclic 1H-Benzimidazole Scaffolds: A Review. BMC Chem. 2019, 13, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prajapat, P.; Kumawat, M.; Talesara, G.L.; Kalal, P.; Agarwal, S.; Kapoor, C.S. Benzimidazole Scaffold as a Versatile Biophore in Drug Discovery: A Review. Chem. Biol. Interface 2018, 8, 1–10. [Google Scholar]
- Nikitina, P.A.; Perevalov, V.P. Methods of synthesis and physicochemical properties of 1-hydroxyimidazoles, imidazole 3-oxides, and their benzoannulated analogs. Chem. Heterocycl. Compd. 2017, 53, 123–149. [Google Scholar] [CrossRef]
- Buján de Vargas, E.I.; Cañas, A.I. From N-n-butyl-2,6-dinitroaniline to a Fused Heterocyclic N-oxide. Tetrahedron Lett. 1996, 37, 767–770. [Google Scholar] [CrossRef]
- Buján, E.I.; Salum, M.L. A simple synthesis of benzimidazole N-oxides from 2-nitroaniline derivatives—Scope and limitations. Can. J. Chem. 2004, 82, 1322–1327. [Google Scholar] [CrossRef]
- Buján, E.I.; Salum, M.L. From N-(dinitrophenyl) amino acids to benzimidazole N-oxides. Synthesis, kinetics and mechanism. J. Phys. Org. Chem. 2006, 19, 187–195. [Google Scholar]
- Politano, F.; Buján, E.I.; Leadbeater, N.E. Preparation of benzimidazole N-oxides by a two-step continuous flow process. Chem. Heterocycl. Compd. 2016, 52, 952–957. [Google Scholar] [CrossRef]
- Politano, F.; Gran-Magano, A.K.; Leadbeater, N.E. One-Pot Two-Step Synthesis of 2-Aryl benzimidazole N-oxides Using Microwave Heating as a Tool. Molecules 2019, 24, 3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politano, F.; Sandoval, A.L.; Uranga, J.G.; Buján, E.I.; Leadbeater, N.E. Using Experimental and Computational Approaches to Probe an Unusual Carbon-Carbon Bond Cleavage Observed in the Synthesis of Benzimidazole N-Oxides. Org. Biomol. Chem. 2021, 19, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Q.; Shreeve, J.M. Fused Heterocycle-Based Energetic Materials (2012–2019). J. Mater. Chem. A 2020, 8, 4193–4216. [Google Scholar] [CrossRef]
- Wu, J.-T.; Xu, J.; Li, W.; Li, H.-B. Coplanar Fused Heterocycle-Based Energetic Materials. Propellants Explos. Pyrotech. 2020, 45, 536–545. [Google Scholar] [CrossRef]
- Tsyshevsky, R.; Smirnov, A.S.; Kuklja, M.M. Comprehensive End-To-End Design of Novel High Energy Density Materials: III. Fused Heterocyclic Energetic Compounds. J. Phys. Chem. C 2019, 123, 8688–8698. [Google Scholar]
- Nitha, P.R.; Soman, S.; John, J. Indole Fused Heterocycles as Sensitizers in Dye-Sensitized Solar Cells: An Overview. Mater. Adv. 2021, 2, 6136–6168. [Google Scholar] [CrossRef]
- Makhova, N.N.; Belen’kii, L.I.; Gazieva, G.A.; Dalinger, I.L.; Konstantinova, L.S.; Kuznetsov, V.V.; Kravchenko, A.N.; Krayushkin, M.M.; Rakitin, O.A.; Starosotnikov, A.M.; et al. Progress in the Chemistry of Nitrogen-, Oxygen- and Sulfur-Containing Heterocyclic Systems. Russ. Chem. Rev. 2020, 89, 55–124. [Google Scholar] [CrossRef]
- Manna, S.K.; Das, T.; Samanta, S. Polycyclic Benzimidazole: Synthesis and Photophysical Properties. ChemistrySelect 2019, 4, 8781–8790. [Google Scholar] [CrossRef]
- Peng, C.-C.; Zhang, M.-J.; Sun, X.-X.; Cai, X.-J.; Chen, Y.; Chen, W.-H. Highly Efficient Anion Transport Mediated by 1,3-Bis(benzimidazol-2-yl)benzene Derivatives Bearing Electron-Withdrawing Substituents. Org. Biomol. Chem. 2016, 14, 8232–8236. [Google Scholar] [CrossRef] [PubMed]
- Annor-Gyamfi, J.K.; Gnanasekaran, K.K.; Bunce, R.A. Syntheses of 1-Aryl-5-nitro-1H-indazoles and a General One-Pot Route to 1-Aryl-1H-indazoles. Molecules 2018, 23, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politano, F.; Gran-Magano, A.K.; Leadbeater, N.E. 2,2′-(1,4-Phenylene)bis(7-nitro-1H-benzimidazole 3-oxide). Molbank 2021, 2021, M1297. https://doi.org/10.3390/M1297
Politano F, Gran-Magano AK, Leadbeater NE. 2,2′-(1,4-Phenylene)bis(7-nitro-1H-benzimidazole 3-oxide). Molbank. 2021; 2021(4):M1297. https://doi.org/10.3390/M1297
Chicago/Turabian StylePolitano, Fabrizio, Ana K. Gran-Magano, and Nicholas E. Leadbeater. 2021. "2,2′-(1,4-Phenylene)bis(7-nitro-1H-benzimidazole 3-oxide)" Molbank 2021, no. 4: M1297. https://doi.org/10.3390/M1297
APA StylePolitano, F., Gran-Magano, A. K., & Leadbeater, N. E. (2021). 2,2′-(1,4-Phenylene)bis(7-nitro-1H-benzimidazole 3-oxide). Molbank, 2021(4), M1297. https://doi.org/10.3390/M1297