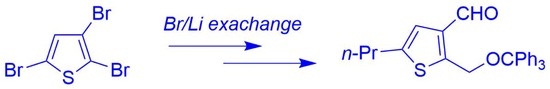
Regioselective Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde
Abstract
:1. Introduction
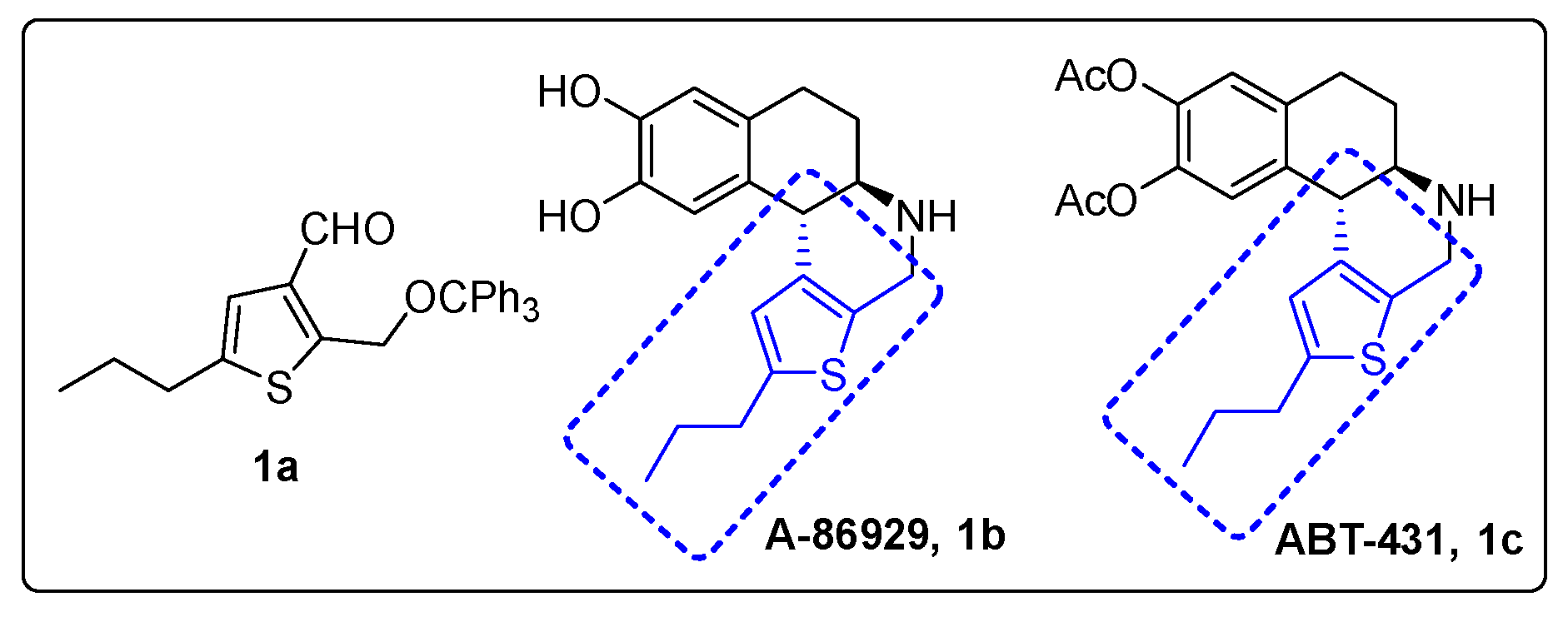
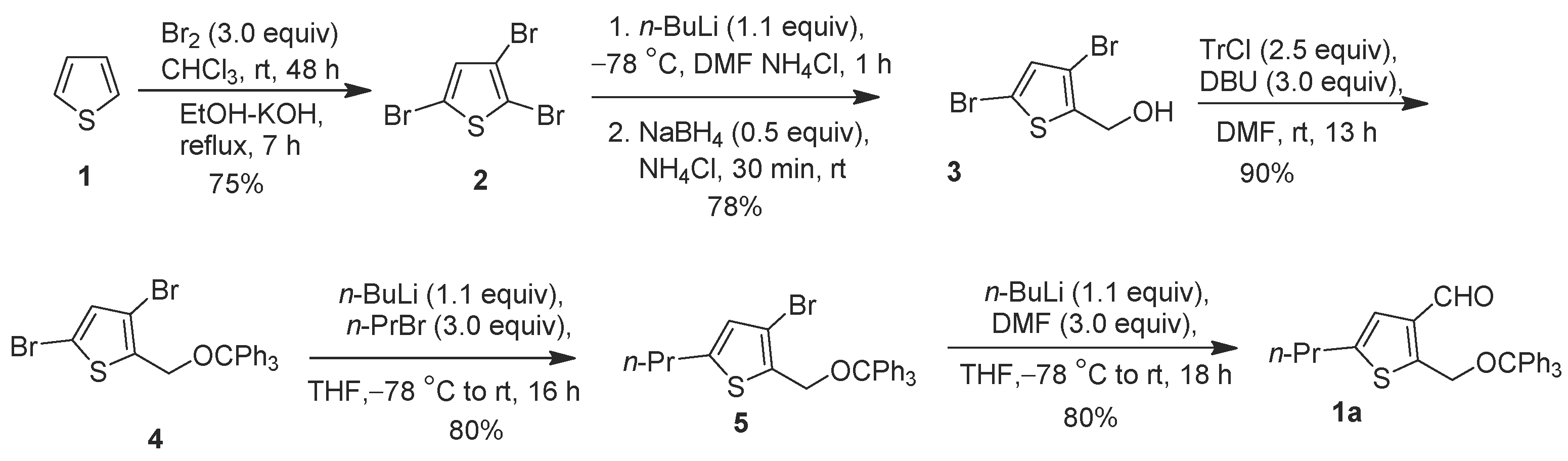
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 2,3,5-Tribromothiophene 2
3.2. Synthesis of (3,5-Dibromothiophen-2-yl)methanol 3
3.3. Synthesis of 3,5-Dibromo-2-((trityloxy)methyl)thiophene 4
3.4. Synthesis of 3-Bromo-5-propyl-2-((trityloxy)methyl)thiophene 5
3.5. Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde 1a
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyodo, K.; Toyama, R.; Hiroki Mori, H.; Yasushi Nishihara, Y. Synthesis and physicochemical properties of piceno[4,3-b:9,10-b′]dithiophene derivatives and their application in organic field-effect transistors. ACS Omega 2017, 2, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Author Huang, P.-Y.; Kim, C.; Chen, M.-C. First tetrabutylanthradithiophene (TBADT) derivatives for solution-processed thin-film transistors. Synlett 2011, 15, 2151–2156. [Google Scholar]
- Nishinaga, S.; Mori, H.; Nishihara, Y. Synthesis and transistor application of bis[1]benzothieno[6,7-d:6′,7′-d′]benzo[1,2-b:4,5-b′]dithiophenes. J. Org. Chem. 2018, 83, 5506–5515. [Google Scholar] [CrossRef] [PubMed]
- Koumura, N.; Wang, Z.S.; Mori, S.; Miyashita, M.; Suzuki, E.; Hara, J.K. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 2006, 128, 14256–14257. [Google Scholar] [CrossRef] [PubMed]
- Thayumanavan, S.; Mendez, J.; Marder, S.R. Synthesis of functionalized organic second-order nonlinear optical chromophores for electro-optic applications. J. Org. Chem. 1999, 64, 4289–4297. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Li, H.; Shu, S.; Dai, A.; Cai, X.; Wang, J.; Yang, D.; Ma, D.; Wang, M.-W.; et al. Discovery of thiophene-containing biaryl amide derivatives as novel glucagon receptor antagonists. Chem. Biol. Drug Des. 2018, 92, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hin, H.; Maita, N.; Thomas, A.G.; Kurosawa, S.; Rojas, C.; Yorita, K.; Barbara, S.S.; Fukui, K.; Tsukamoto, T. Structural basis for potent inhibition of D-amino acid oxidase bythiophene carboxylic acids. Eur. J. Med. Chem. 2018, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamada, K.; Tomioka, K. Construction of arene-fused-piperidine motifs by asymmetric addition of 2-trityloxymethylaryllithiums to nitroalkenes: The asymmetric synthesis of a dopamine D1 full agonist, A-86929. J. Am. Chem. Soc. 2004, 126, 1954–1955. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.; Hajra, S. Catalytic enantioselective synthesis of A-86929, a dopamine D1 agonist. Chem. Commun. 2011, 47, 3981–3982. [Google Scholar]
- Altinok, E.; Frausto, F.; Thomas III, S.W. Water-soluble fluorescent polymers that respond to singlet oxygen. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2526–2535. [Google Scholar] [CrossRef]
- Garnier, F. Functionalized conducting polymer. Angew. Chem. Int. Ed. Engl. 1989, 28, 513–517. [Google Scholar] [CrossRef]
- Han, P.; Yang, Z.; Cao, H.; He, W.; Wang, D.; Zhang, J.; Xing, Y.; Gao, H. Nonlinear optical properties of the novel kind of organic donor-acceptor thiophene derivatives with click chemistry modification. Tetrahedron 2017, 73, 6210–6216. [Google Scholar] [CrossRef]
- Russel, K.R.; Press, B.J.; Rampulla, A.R. Thiophene system 9, thienopyrimidinedione derivatives as potential antihypertensive agent. J. Med. Chem. 1988, 31, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, S.; Sing, I.; Saxena, K.K.; Kumar, A. Synthesis, characterization and biological activity of various substituted benzothiazol derivatives. Dig. J. Nanomater. Bios. 2010, 5, 67–76. [Google Scholar]
- Badar, S.M.I. Synthesis and anti-inflammatory activity of novel 2,5-disubstituted thiophene derivatives. Turk. J. Chem. 2001, 35, 131–143. [Google Scholar]
- Gronowitz, S.; Raznikiewicz, T. 3-Bromothiophene. Org. Synth. 1964, 44, 9. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar, S. Regioselective Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde. Molbank 2021, 2021, M1289. https://doi.org/10.3390/M1289
Bar S. Regioselective Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde. Molbank. 2021; 2021(4):M1289. https://doi.org/10.3390/M1289
Chicago/Turabian StyleBar, Sukanta. 2021. "Regioselective Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde" Molbank 2021, no. 4: M1289. https://doi.org/10.3390/M1289
APA StyleBar, S. (2021). Regioselective Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde. Molbank, 2021(4), M1289. https://doi.org/10.3390/M1289