Novel Synthesis of N-Acetylcysteine Medicine Using an Effective Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
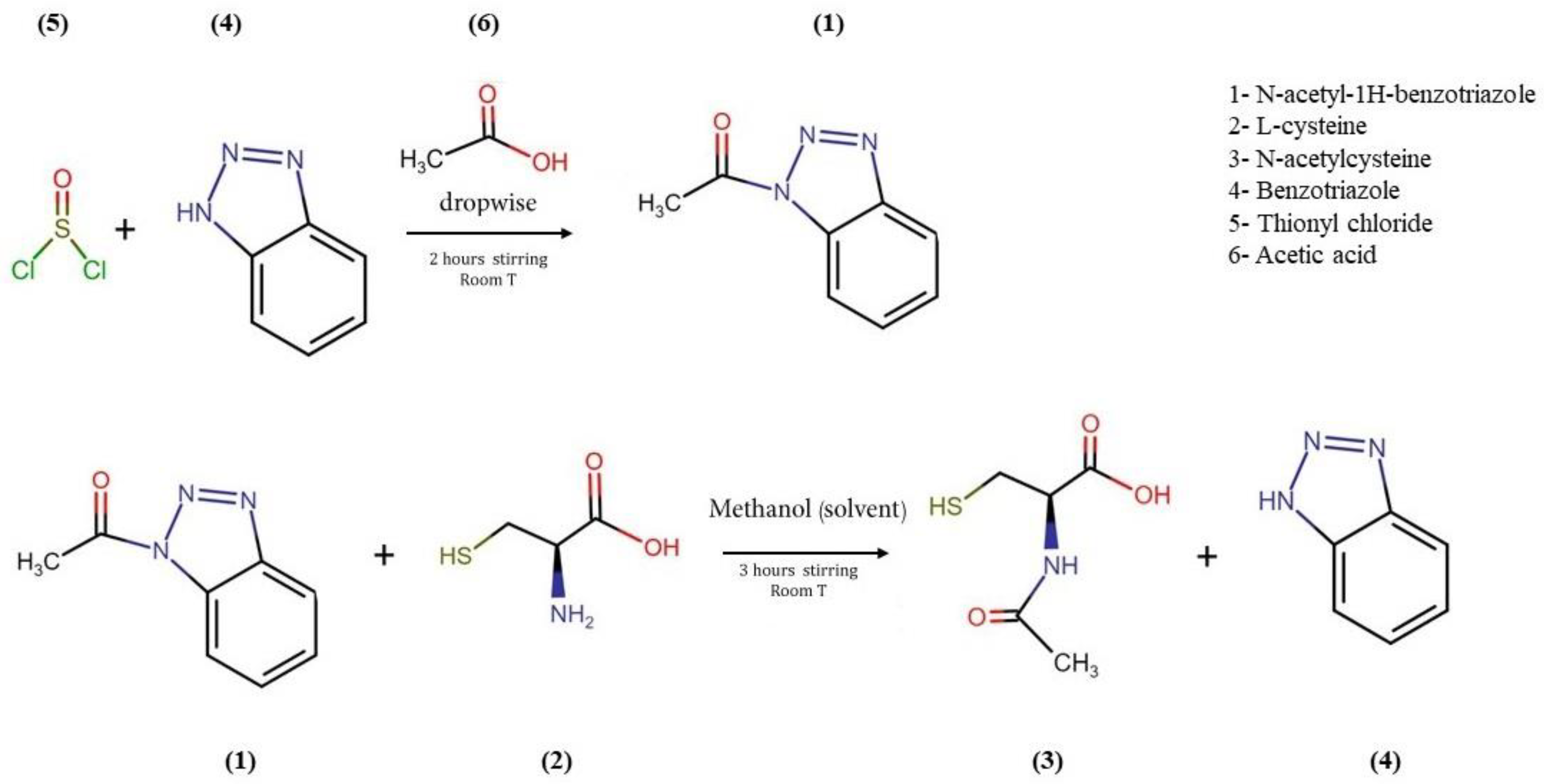
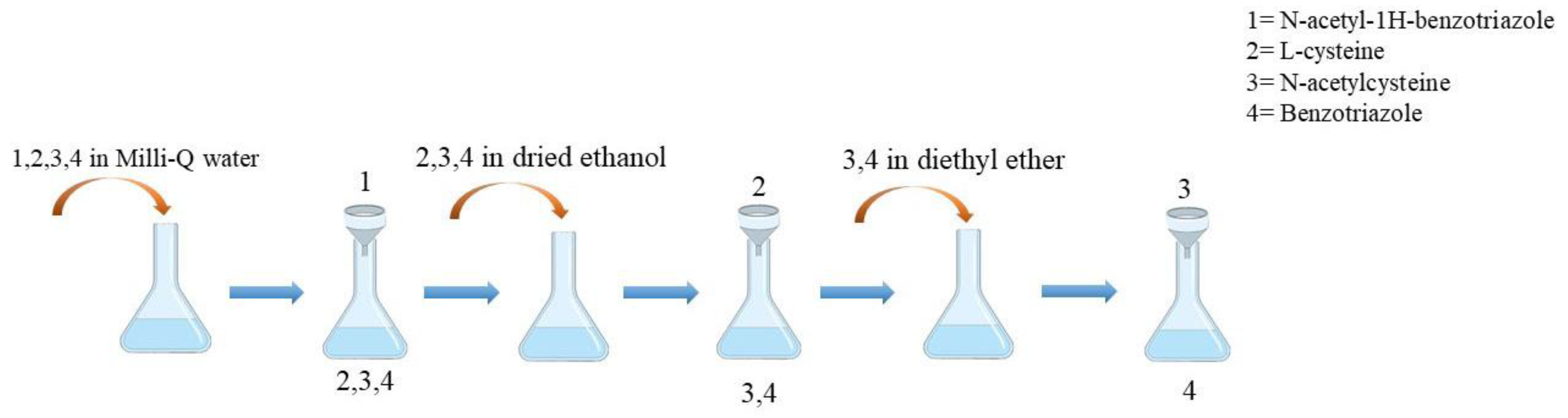
2.2.1. Novel Chemical Synthesis of N-Acetylcysteine
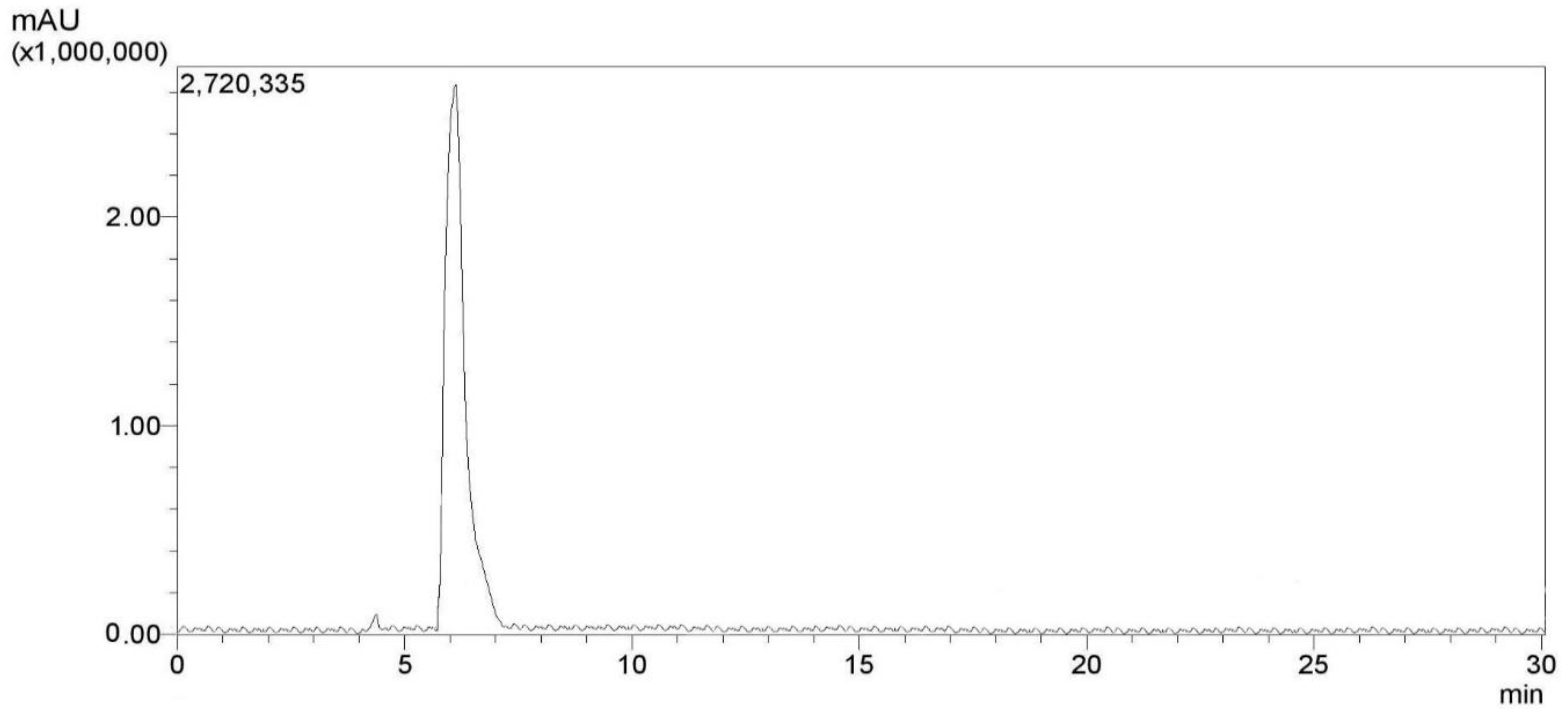
2.2.2. Characterization Method
3. Results and Discussion
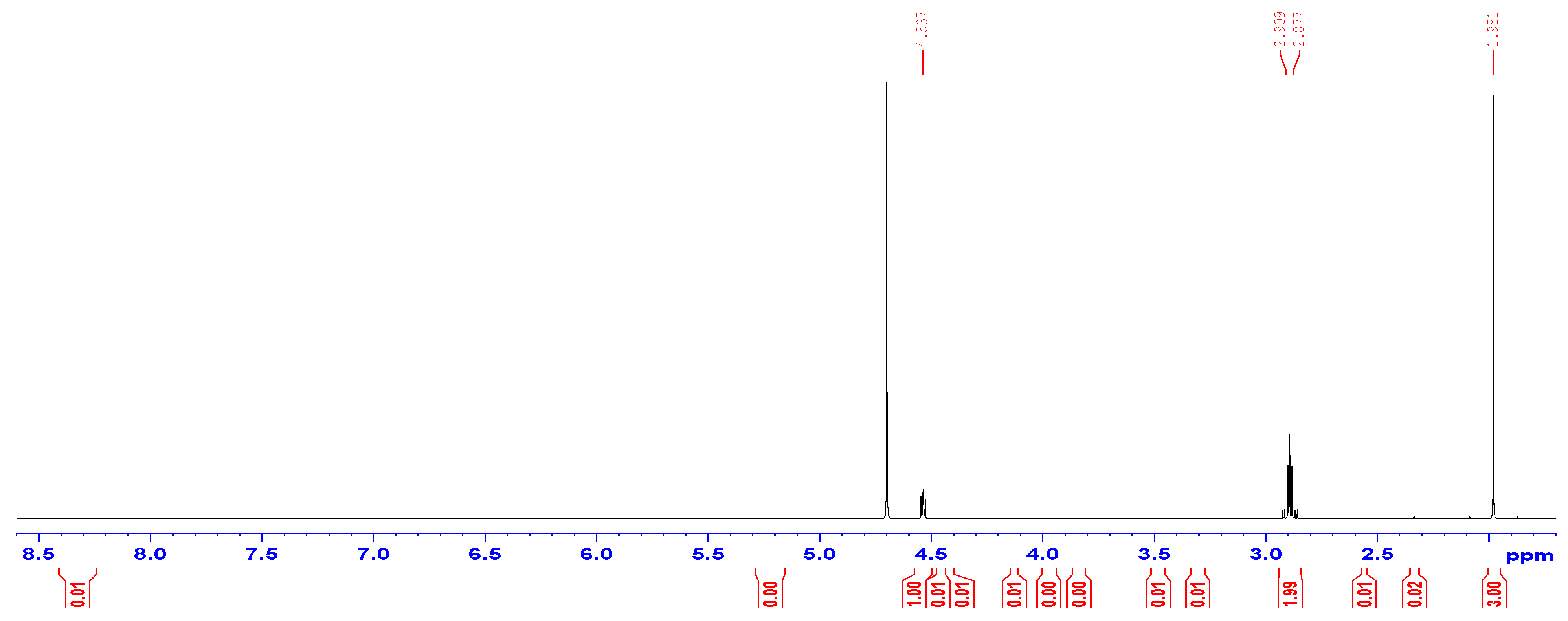
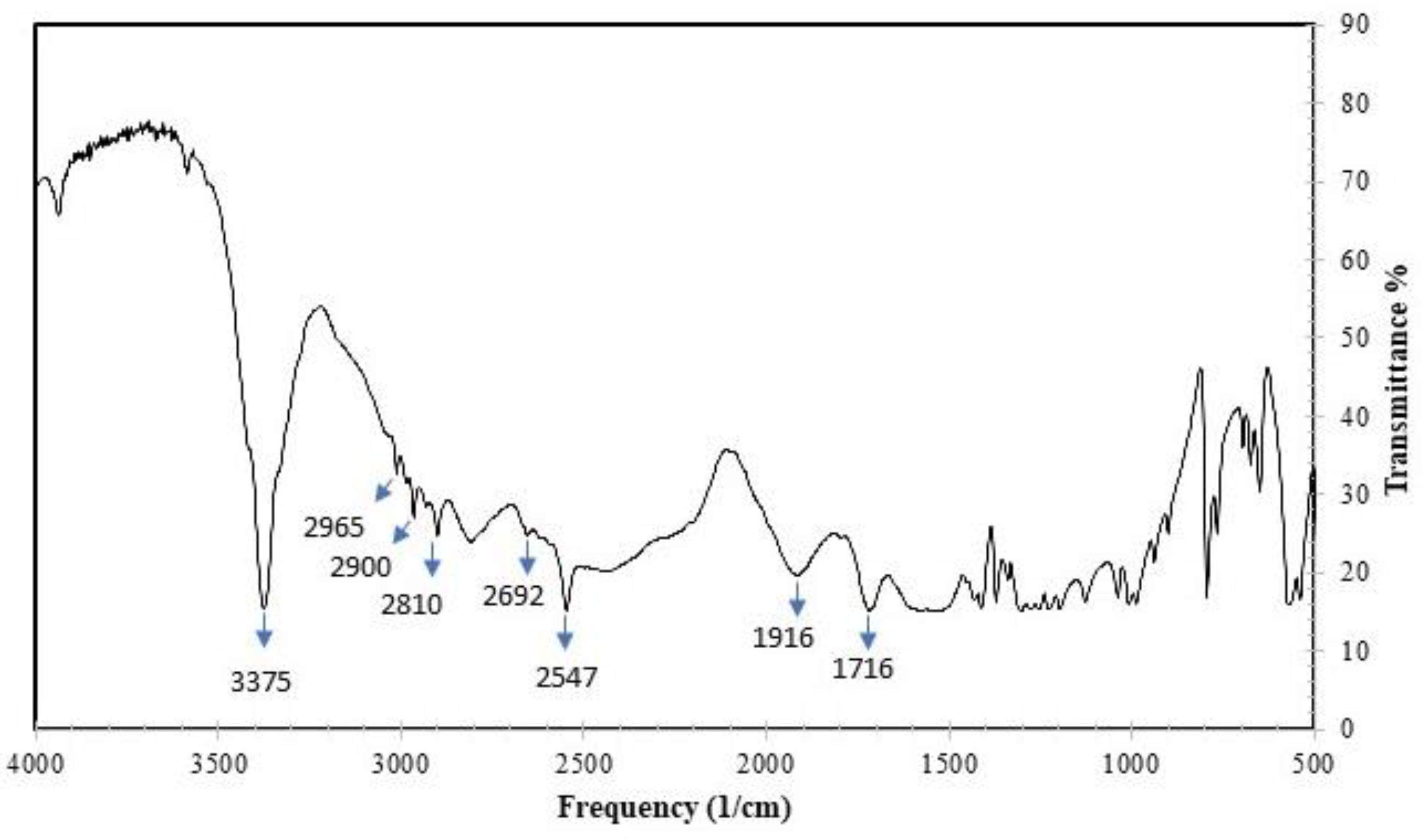
3.1. Characterization of the Synthesized N-Acetylcysteine
3.2. Alternative Synthesis Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetty, R.; Udupa, N.; Mutalik, S.; Kulkarni, V.; Rao, V. Mechanisms and Therapeutics of N-acetylcysteine: A Recent Update. Res. J. Pharm. Technol. 2019, 12, 2584. [Google Scholar] [CrossRef]
- Bauerlein, D.K.; Akbar, H.N.; von Rosenvinge, E.C.; Loughry, N.D.; John, P.R. Benefit of N-Acetylcysteine in Postoperative Hepatic Dysfunction: Case Report and Review of Literature. Case Rep. Hepatol. 2019, 2019, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carbonell, N.; Sanjuán, R.; Blasco, M.; Jordá, Á.; Miguel, A. N-acetylcysteine: Short-Term Clinical Benefits After Coronary Angiography in High-Risk Renal Patients. Rev. Española Cardiol. Engl. Ed. 2010, 63, 12–19. [Google Scholar] [CrossRef]
- Koh, A.S.; Simmons-Willis, T.A.; Pritchard, J.B.; Grassl, S.M.; Ballatori, N. Identification of a Mechanism by Which the Methylmercury Antidotes N-Acetylcysteine and Dimercaptopropanesulfonate Enhance Urinary Metal Excretion: Transport by the Renal Organic Anion Transporter-1. Mol. Pharmacol. 2002, 62, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; McCormack, S. Acetylcysteine for Patients Requiring Mucous Secretion Clearance: A Review of Clinical Effectiveness and Safety; Ottawa, Report; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 14 June 2019.
- Liu, Y.; Wang, M.; Luo, G.; Qian, X.; Wu, C.; Zhang, Y.; Chen, B.; Leung, E.L.H.; Tang, Y. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: A case report. Medicine 2020, 99, 22577. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Perl, A.; Smith, D.; Lewis, T.; Kon, Z.; Goldenberg, R.; Yarta, K.; Staniloae, C.; Williams, M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol. 2020, 219, 108544. [Google Scholar] [CrossRef]
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020, 34, 13185–13193. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; Mccord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Kesarwala, A.H.; Krishna, M.C.; Mitchell, J.B. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Gibson, K.R.; Neilson, I.L.; Barrett, F.; Winterburn, T.J.; Sharma, S.; MacRury, S.M.; Megson, I.L. Evaluation of the Antioxidant Properties of N-acetylcysteine in Human Platelets: Prerequisite for Bioconversion to Glutathione for Antioxidant and Antiplatelet Activity. J. Cardiovasc. Pharmacol. 2009, 54, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Opina, A.; Sail, D.; Blackman, B.; Saito, K.; Brender, J.R.; Malinowski, R.M.; Seki, T.; Oshima, N.; Crooks, D.R.; et al. Real-Time insight into in vivo redox status utilizing hyperpolarized [1-13C] N-acetyl cysteine. Sci. Rep. 2021, 11, 12155. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, F.; Ziaee, M.; Taseidifar, M. Synthesis and application of a green surfactant for the treatment of water containing PFAS/ hazardous metal ions. J. Hazard. Mater. 2021, 407, 124800. [Google Scholar] [CrossRef]
- Taseidifar, M. Environmental applications of a biodegradable cysteine-based surfactant. Ecotoxicol. Environ. Saf. 2020, 206, 111389. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Zhang, Y.; Singh, S.K. Efficient Conversion of Carboxylic Acids into N-Acylbenzotriazoles. Synthesis 2003, 18, 2795–2798. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Synthesis of Essential Drugs, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 313–314. [Google Scholar]
- Riera, A.A.; Leguey, V.M.; Garcia, V.G.; Garcia, J.G. Universidad de Alicante. Process for the Electrochemical Synthesis of n-acetylcysteine from Cystine. U.S. Patent No. 6,159,352, 12 December 2000. [Google Scholar]
- Martin, T.A.; Waller, C.W. Process for the N-Monoacylation of Cysteine. USA Patent No. 2,029,488, 18 May 1965. [Google Scholar]
- Katritzky, A.R.; Tala, S.R.; Abo-Dya, N.E.; Gyanda, K.; El-Gendy, B.E.D.M.; Abdel-Samii, Z.K.; Steel, P.J. Selective Synthesis and Structural Elucidation of S-Acyl- and N-Acylcysteines. J. Org. Chem. 2009, 74, 7165–7167. [Google Scholar] [CrossRef] [PubMed]
- Pashley, R.M.; Taseidifar, M. Method for Acylating Amino Acids and Uses of N_acyl Amino Acid Products. PCT Patent PCT/AU2020/050142, 18 February 2020. [Google Scholar]
| Sample | Weight (mg) | C% | N% | H% | S% |
|---|---|---|---|---|---|
| Test 1 | 1.17 | 37.0 | 8.5 | 5.5 | 20.8 |
| Test 2 | 1.38 | 37.1 | 8.6 | 5.6 | 20.9 |
| Expected values | 36.8 | 8.6 | 5.5 | 19.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaee, F.; Ziaee, M. Novel Synthesis of N-Acetylcysteine Medicine Using an Effective Method. Molbank 2021, 2021, M1288. https://doi.org/10.3390/M1288
Ziaee F, Ziaee M. Novel Synthesis of N-Acetylcysteine Medicine Using an Effective Method. Molbank. 2021; 2021(4):M1288. https://doi.org/10.3390/M1288
Chicago/Turabian StyleZiaee, Farzaneh, and Mohammad Ziaee. 2021. "Novel Synthesis of N-Acetylcysteine Medicine Using an Effective Method" Molbank 2021, no. 4: M1288. https://doi.org/10.3390/M1288
APA StyleZiaee, F., & Ziaee, M. (2021). Novel Synthesis of N-Acetylcysteine Medicine Using an Effective Method. Molbank, 2021(4), M1288. https://doi.org/10.3390/M1288





