3-[N-(4-Methoxybenzyl)amino]benzo[de]anthracen-7-one
Abstract
:1. Introduction
2. Results and Discussion
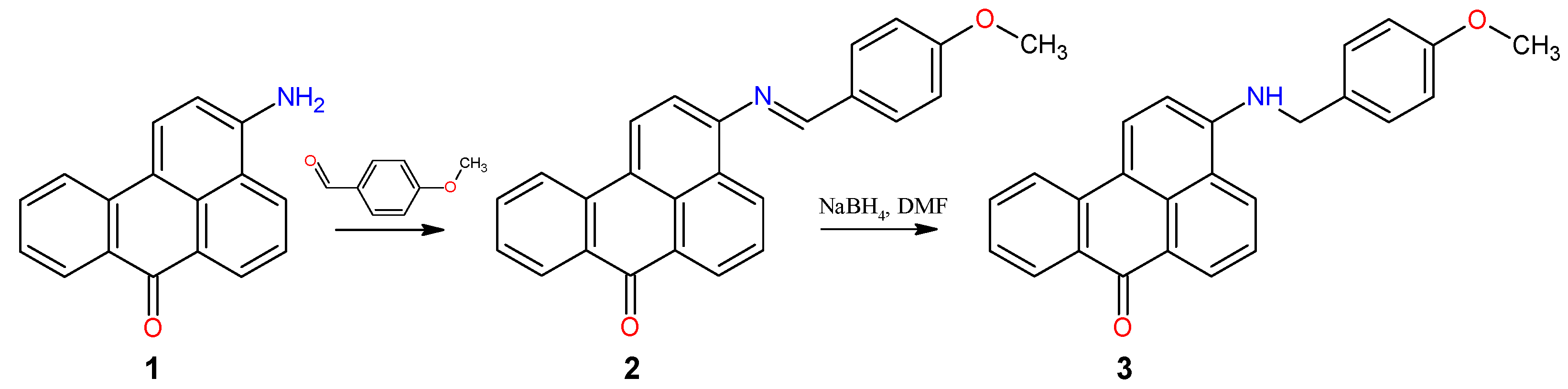
2.1. Synthesis
2.2. Spectroscopic Properties
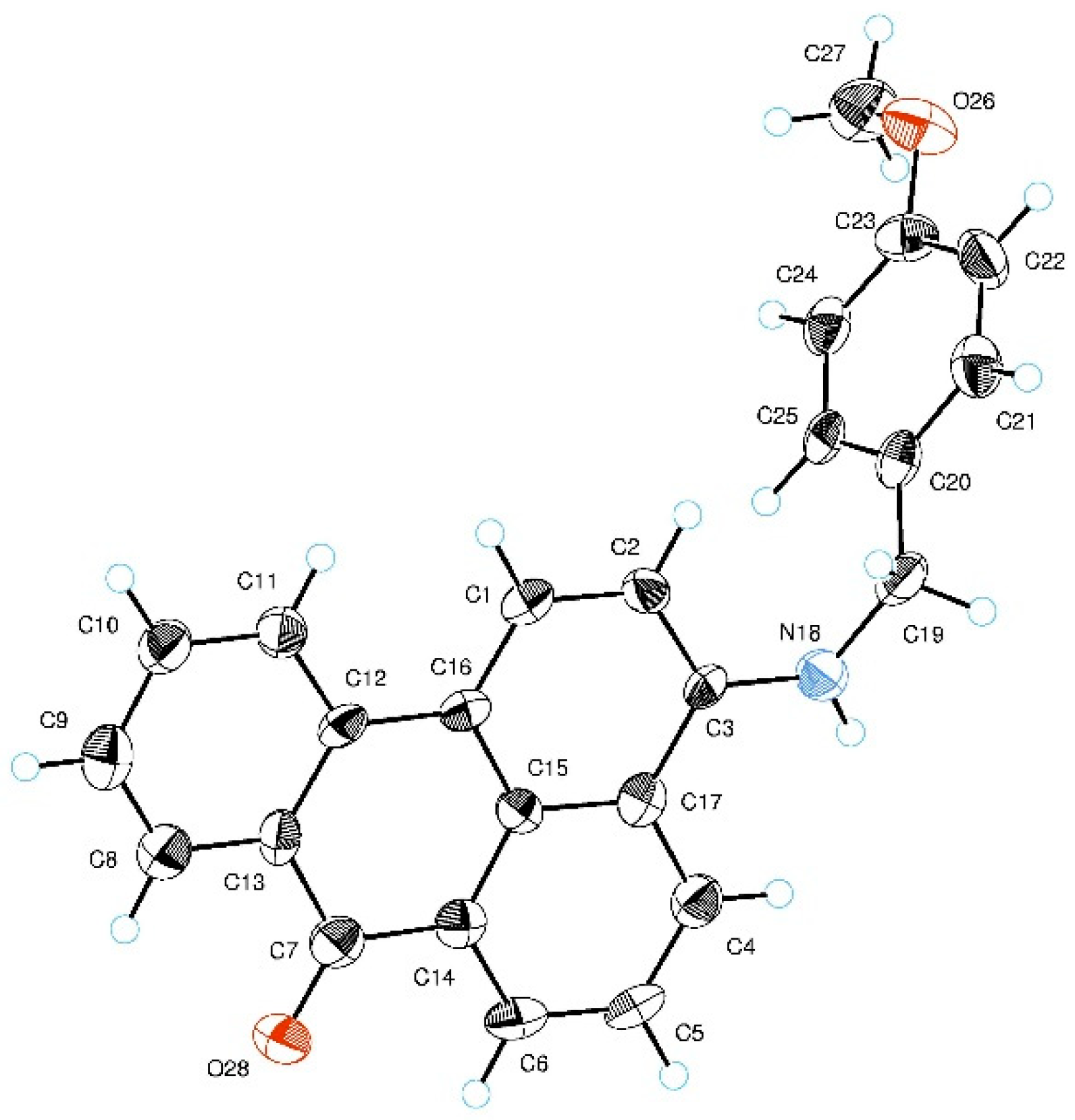
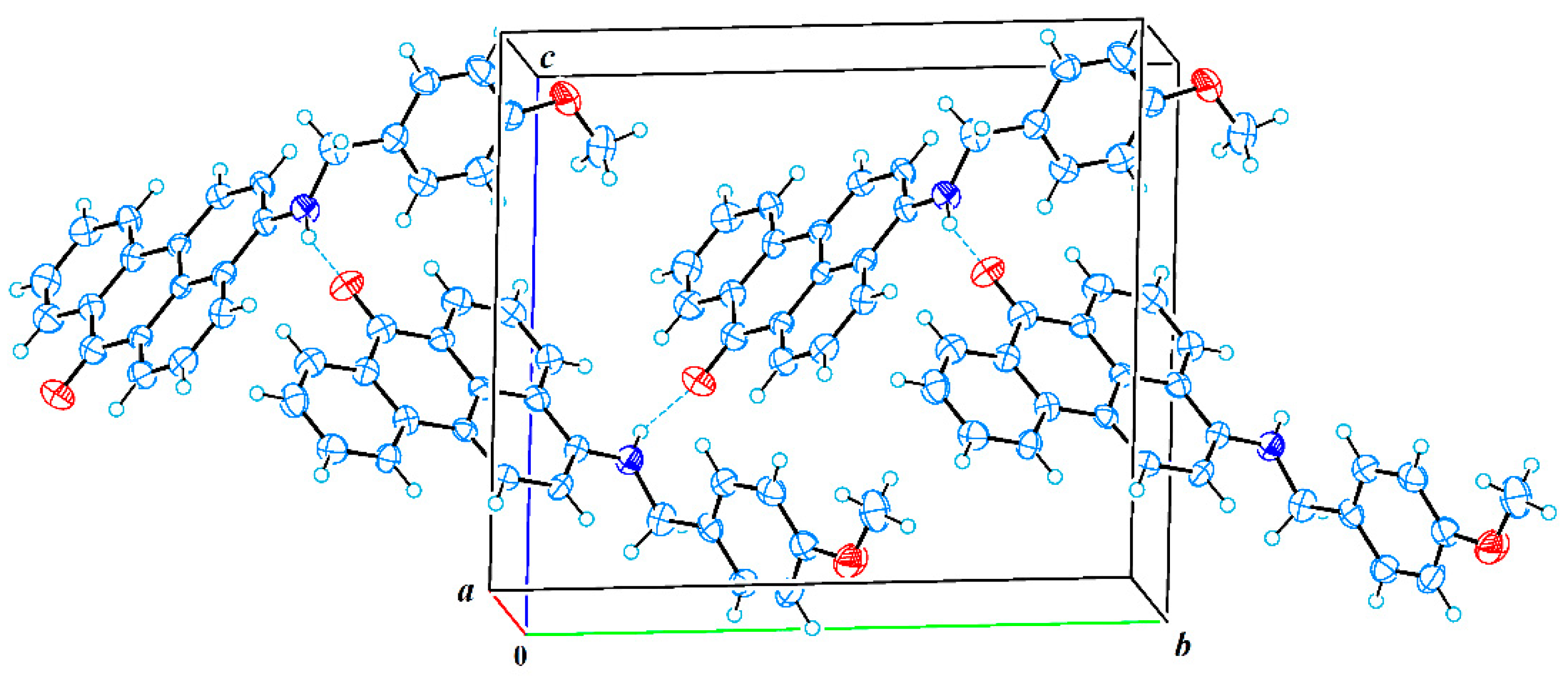
2.3. X-ray Crystallographic Study
3. Materials and Methods
3.1. Materials and Basic Measurements
3.2. Synthesis and Characterization
3.2.1. 3-[N-(4-Methoxybenzyledene)amino]benzo[de]anthracen-7-one (2)
3.2.2. 3-[N-(4-Methoxybenzyl)amino]benzo[de]anthracen-7-one (3)
3.3. Single-Crystal X-ray Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grabchev, I.; Moneva, I. Synthesis and properties of benzanthrone derivatives as luminophore dyes for liquid crystals. Dyes Pigment. 1998, 37, 155–164. [Google Scholar] [CrossRef]
- Grabchev, I.; Bojinov, V.; Moneva, I. Functional properties of azomethine substituted benzanthrone dyes for use in nematic liquid crystals. J. Mol. Struct. 1998, 471, 19–25. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Wolarz, E.; Bauman, D.; Stoyanov, S. Spectral properties of 3-benzanthrone derivative dyes in isotropic solvents, polymer film and liquid crystal. Z. Naturforsch. A 2001, 56, 291–296. [Google Scholar] [CrossRef]
- Tang, P.; Wang, H.; Zhang, W.; Chen, F.-E. Asymmetric catalytic hydrogenation of imines and enamines in natural product synthesis. Green Synth. Catal. 2020, 1, 26–41. [Google Scholar] [CrossRef]
- Orlova, N.; Nikolajeva, I.; Pučkins, A.; Belyakov, S.; Kirilova, E. Heterocyclic schiff bases of 3-Aminobenzanthrone and their reduced analogues: Synthesis, properties and spectroscopy. Molecules 2021, 26, 2570. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, E.; Mickevica, I.; Mezaraupe, L.; Puckins, A.; Rubenina, I.; Osipovs, S.; Kokina, I.; Bulanovs, A.; Kirjusina, M.; Gavarane, I. Novel dye for detection of callus embryo by confocal laser scanning fluorescence microscopy. Luminescence 2019, 34, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Gavarane, I.; Kirilova, J.; Rubenina, I.; Mezaraupe, L.; Deksne, G.; Puckins, A.; Kokina, I.; Bulanovs, A.; Kirjusina, M.; Osipovs, S. A simple and rapid staining technique for sex determination of Trichinella larvae parasites by confocal laser scanning microscopy. Microsc. Microanal. 2019, 25, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Tarabara, U.; Kirilova, E.; Kirilov, G.; Vus, K.; Zhytniakivska, O.; Trusova, V.; Gorbenko, G. Benzanthrone dyes as mediators of cascade energy transfer in insulin amyloid fibrils. J. Mol. Liq. 2021, 324, 115102. [Google Scholar] [CrossRef]
- Itsuno, S. Boron hydride reduction. ACS Symp. Ser. 2016, 1236, 241–274. [Google Scholar]
- Krasovitskii, B.M.; Bolotin, B.M. Organic Luminescent Materials; Wiley-VCH: New York, NY, USA, 1988; pp. 149–152. [Google Scholar]
- Lüttringhaus, A.; Neresheimer, H. Zur Kenntnis des Benzanthrons. Eur. J. Org. Chem. 1929, 473, 259–289. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A Found. Cryst. 2008, 64, 112–122. [Google Scholar]
| Solvent | Absorption λabs (lgε), nm | Fluorescence λem, nm | ||
|---|---|---|---|---|
| Imine 2 | Amine 3 | Imine 2 | Amine 3 | |
| Benzene | 429 (4.58) | 482 (4.66) | 561 | 567 |
| Ethyl acetate | 425 (4.58) | 491 (4.69) | 581 | 588 |
| Chloroform | 434 (4.59) | 489 (4.70) | 500 | 600 |
| Acetone | 428 (4.58) | 502 (4.77) | 500 | 612 |
| DMF | 434 (4.59) | 514 (4.72) | 500 | 621 |
| DMSO | 437 (4.62) | 524 (4.73) | 511 | 635 |
| Ethanol | 436 (4.32) | 520 (4.64) | 540 | 658 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirilova, A.; Pučkins, A.; Belyakov, S.; Kirilova, E. 3-[N-(4-Methoxybenzyl)amino]benzo[de]anthracen-7-one. Molbank 2021, 2021, M1287. https://doi.org/10.3390/M1287
Kirilova A, Pučkins A, Belyakov S, Kirilova E. 3-[N-(4-Methoxybenzyl)amino]benzo[de]anthracen-7-one. Molbank. 2021; 2021(4):M1287. https://doi.org/10.3390/M1287
Chicago/Turabian StyleKirilova, Alise, Aleksandrs Pučkins, Sergey Belyakov, and Elena Kirilova. 2021. "3-[N-(4-Methoxybenzyl)amino]benzo[de]anthracen-7-one" Molbank 2021, no. 4: M1287. https://doi.org/10.3390/M1287
APA StyleKirilova, A., Pučkins, A., Belyakov, S., & Kirilova, E. (2021). 3-[N-(4-Methoxybenzyl)amino]benzo[de]anthracen-7-one. Molbank, 2021(4), M1287. https://doi.org/10.3390/M1287






