8-[4-(2-Hydroxypropane-2-yl)phenyl]-1,3,4,4,5,7-hexamethyl-4-boron-3a,4a-diaza-S-indacene
Abstract
:1. Introduction
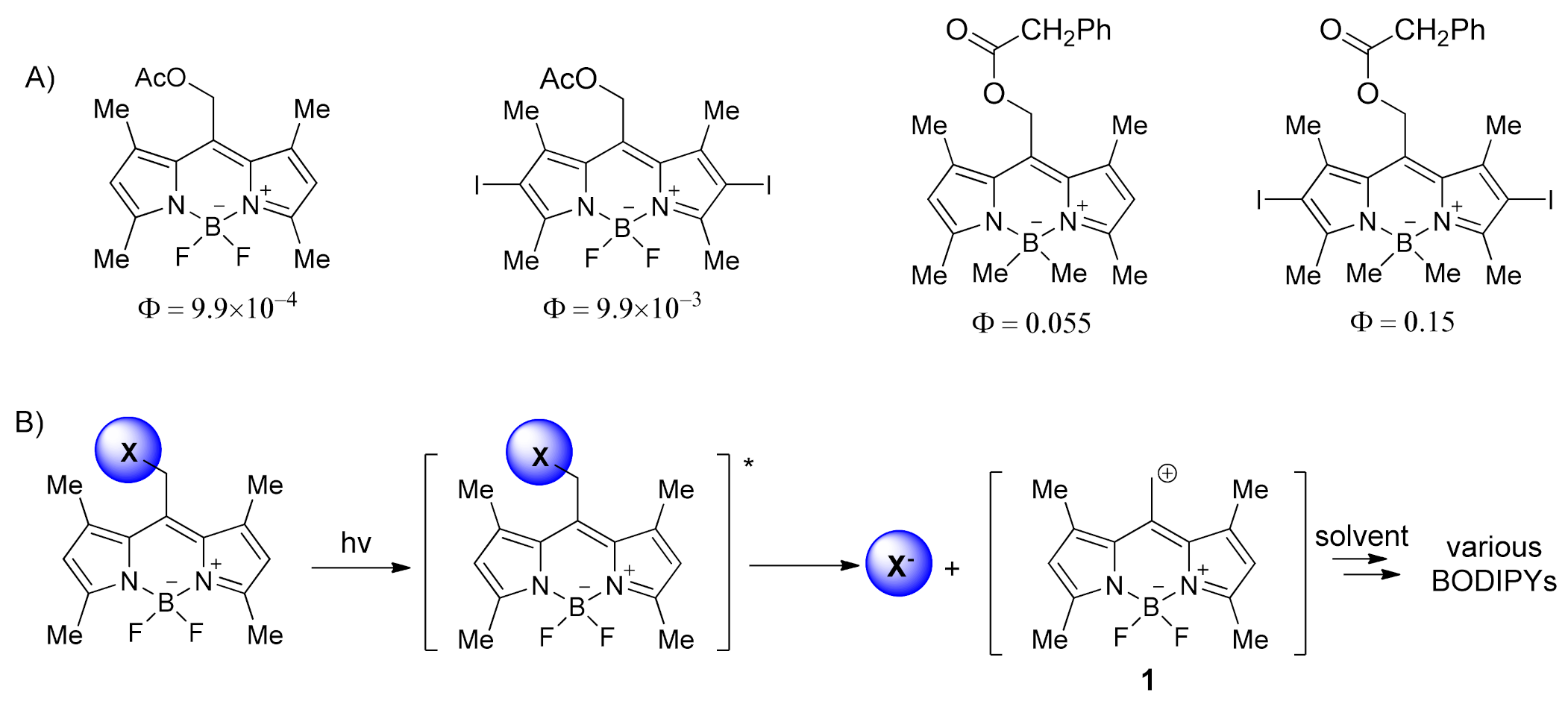
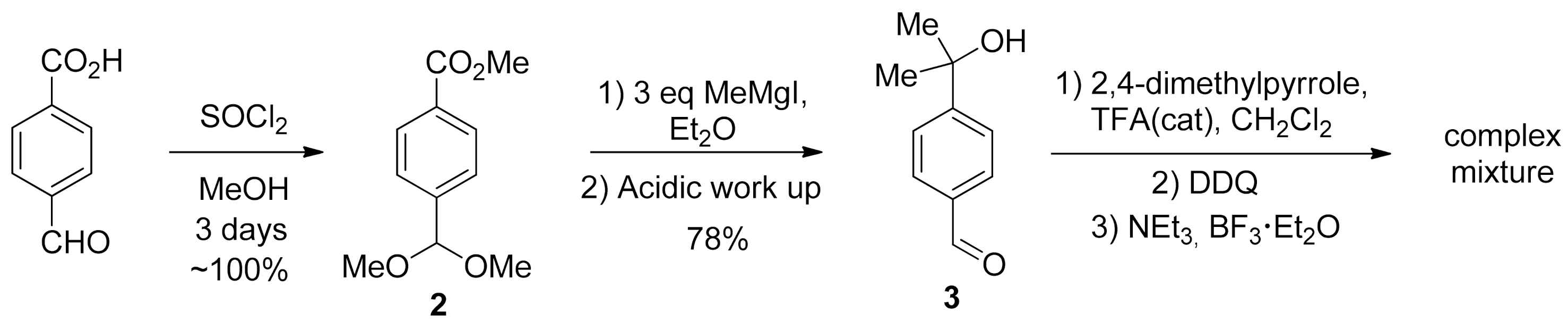
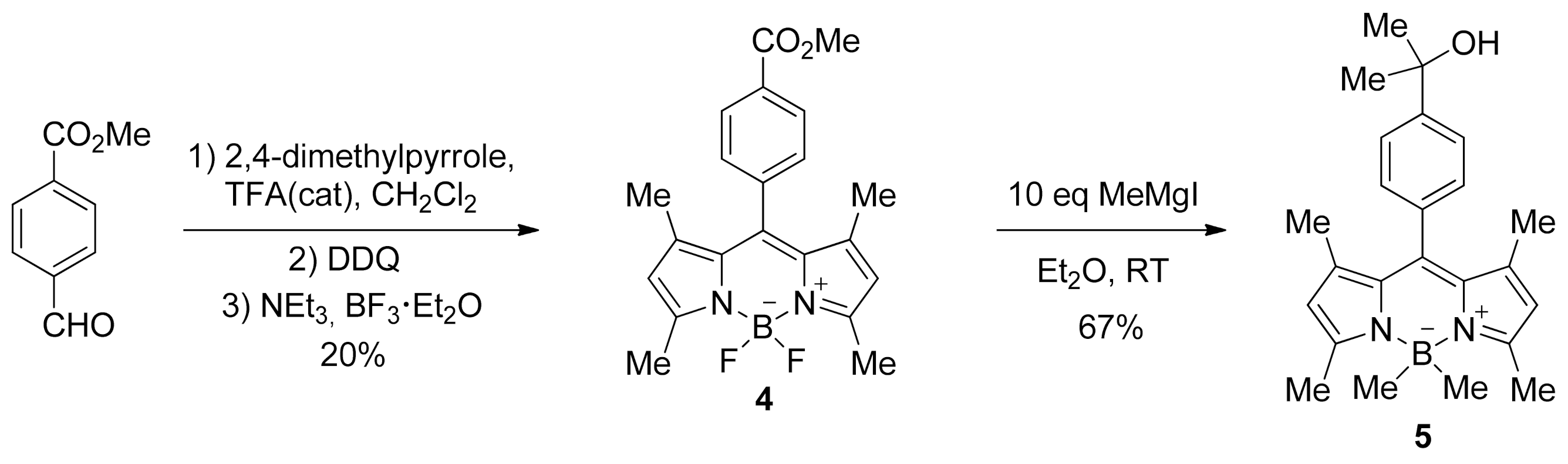
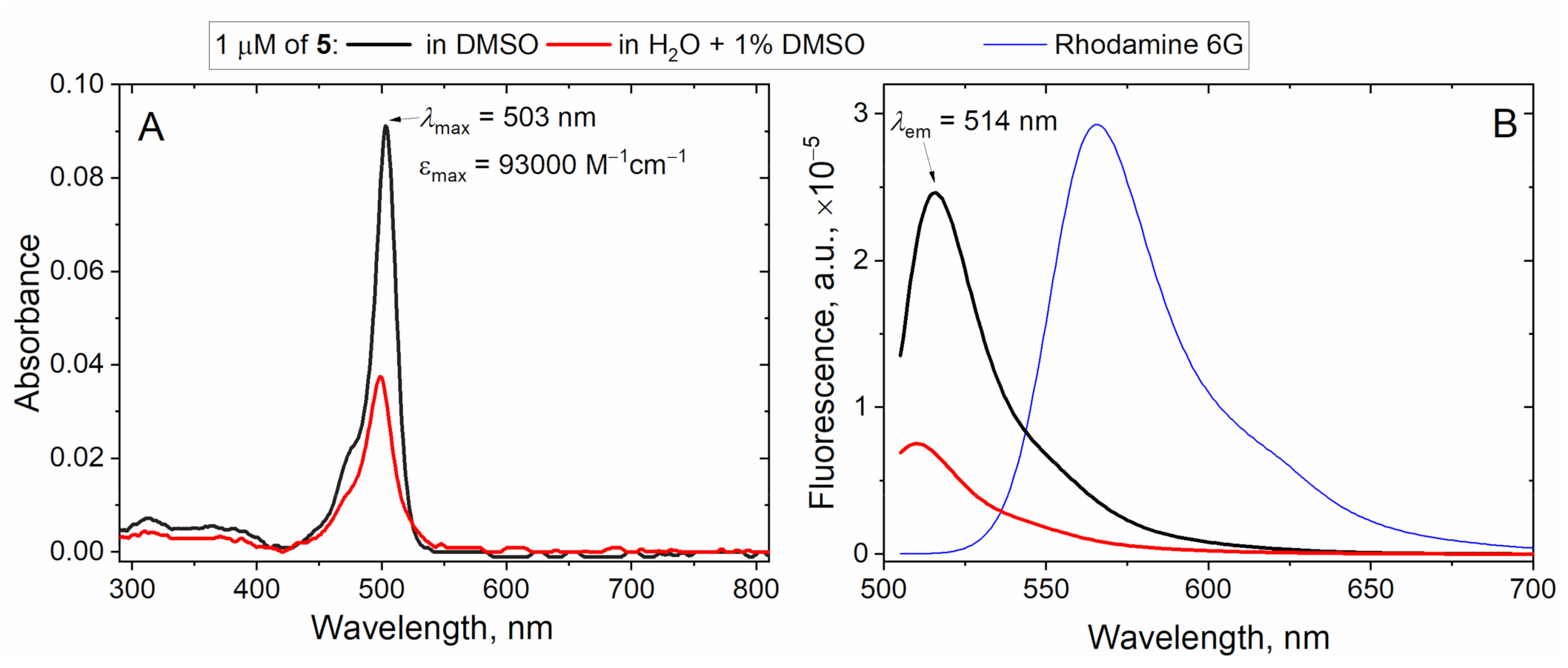
2. Results and Discussion
3. Materials and Methods
- Methyl 4-(dimethoxymethyl)benzoate (2).
- 4-(2-hydroxypropan-2-yl)benzaldehyde (3).
- Interaction of aldehyde 2 with 2,4-dimethylpyrrole.
- 4,4-difluoro-8-[4-(methoxycarbonyl)phenyl]-1,3,5,7-tetramethyl-4-boron-3a,4a-diaza-s-indacene (4).
- 8-[4-(2-hydroxypropane-2-yl)phenyl]-1,3,4,4,5,7-hexamethyl-4-boron-3a,4a-diaza-s-indacene (5).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Shen, Z. Editorial: BODIPYs and Their Derivatives: The Past, Present and Future. Front. Chem. 2020, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.P.; Syed, A.; Beck, C.L.; Albright, T.R.; Mahoney, K.M.; Unash, R.; Smith, E.A.; Winter, A.H. BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light. J. Am. Chem. Soc. 2015, 137, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.; Liu, P.; Miller, E.W.; Weinstain, R. Meso -Methylhydroxy BODIPY: A Scaffold for Photo-Labile Protecting Groups. Chem. Commun. 2015, 51, 6369–6372. [Google Scholar] [CrossRef] [PubMed]
- Weinstain, R.; Slanina, T.; Kand, D.; Klán, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef] [PubMed]
- Goeldner, M.; Givens, R. Dynamic Studies in Biology: Phototriggers, Photoswitches and Caged Biomolecules; Wiley-VCH: Weinheim, Germany, 2005; ISBN 978-3-527-30783-8. [Google Scholar]
- Vorobev, A.Y.; Moskalensky, A.E. Long-Wavelength Photoremovable Protecting Groups: On the Way to in Vivo Application. Comput. Struct. Biotechnol. J. 2020, 18, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Slanina, T.; Shrestha, P.; Palao, E.; Kand, D.; Peterson, J.A.; Dutton, A.S.; Rubinstein, N.; Weinstain, R.; Winter, A.H.; Klán, P. In Search of the Perfect Photocage: Structure–Reactivity Relationships in Meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139, 15168–15175. [Google Scholar] [CrossRef] [PubMed]
- Sitkowska, K.; Hoes, M.F.; Lerch, M.M.; Lameijer, L.N.; van der Meer, P.; Szymański, W.; Feringa, B.L. Red-Light-Sensitive BODIPY Photoprotecting Groups for Amines and Their Biological Application in Controlling Heart Rhythm. Chem. Commun. 2020, 56, 5480–5483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Chinapang, P.; Iwami, H.; Enomoto, T.; Akai, T.; Kondo, M.; Masaoka, S. Dirhodium-Based Supramolecular Framework Catalyst for Visible-Light-Driven Hydrogen Evolution. Inorg. Chem. 2021, 60, 12634–12643. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.Y.; Trofimov, B.A.; Mikhaleva, A.I.; Zorina, N.V.; Protzuk, N.I.; Petrushenko, K.B.; Ushakov, I.A.; Dvorko, M.Y.; Méallet-Renault, R.; Clavier, G.; et al. Synthesis and Optical Properties of 2-(Benzo[b]Thiophene-3-Yl)Pyrroles and a New BODIPY Fluorophore (BODIPY=4,4-Difluoro-4-Bora-3a,4a-Diaza-s-Indacene). Chem.–A Eur. J. 2009, 15, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Ashokkumar, P.; Shen, Z.; Rurack, K. On the Aggregation Behaviour and Spectroscopic Properties of Alkylated and Annelated Boron-Dipyrromethene (BODIPY) Dyes in Aqueous Solution. ChemPhotoChem 2020, 4, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Marfin, Y.S.; Banakova, E.A.; Merkushev, D.A.; Usoltsev, S.D.; Churakov, A.V. Effects of Concentration on Aggregation of BODIPY-Based Fluorescent Dyes Solution. J. Fluoresc. 2020, 30, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Al-Temeem, N.; Jameel, S.; Abd Alfahdawi, A. The Spectroscopic Behaviour of Rhodamine 6G in Liquid and Solid Solutions. Baghdad Sci. J. 2008, 5, 107–114. [Google Scholar]
- Zoller, B.; Zapp, J.; Huy, P.H. Rapid Organocatalytic Formation of Carbon Monoxide: Application towards Carbonylative Cross Couplings. Chem.–A Eur. J. 2020, 26, 9632–9638. [Google Scholar] [CrossRef] [PubMed]
- Barrios, F.J.; Springer, B.C.; Colby, D.A. Control of Transient Aluminum–Aminals for Masking and Unmasking Reactive Carbonyl Groups. Org. Lett. 2013, 15, 3082–3085. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zobnina, A.; Moskalensky, A.; Vorob’ev, A. 8-[4-(2-Hydroxypropane-2-yl)phenyl]-1,3,4,4,5,7-hexamethyl-4-boron-3a,4a-diaza-S-indacene. Molbank 2021, 2021, M1286. https://doi.org/10.3390/M1286
Zobnina A, Moskalensky A, Vorob’ev A. 8-[4-(2-Hydroxypropane-2-yl)phenyl]-1,3,4,4,5,7-hexamethyl-4-boron-3a,4a-diaza-S-indacene. Molbank. 2021; 2021(4):M1286. https://doi.org/10.3390/M1286
Chicago/Turabian StyleZobnina, Anastasiya, Alexander Moskalensky, and Aleksey Vorob’ev. 2021. "8-[4-(2-Hydroxypropane-2-yl)phenyl]-1,3,4,4,5,7-hexamethyl-4-boron-3a,4a-diaza-S-indacene" Molbank 2021, no. 4: M1286. https://doi.org/10.3390/M1286
APA StyleZobnina, A., Moskalensky, A., & Vorob’ev, A. (2021). 8-[4-(2-Hydroxypropane-2-yl)phenyl]-1,3,4,4,5,7-hexamethyl-4-boron-3a,4a-diaza-S-indacene. Molbank, 2021(4), M1286. https://doi.org/10.3390/M1286





