3-(3-Hydroxypropyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxaldehyde Methyl Hemiacetal
Abstract
:1. Introduction
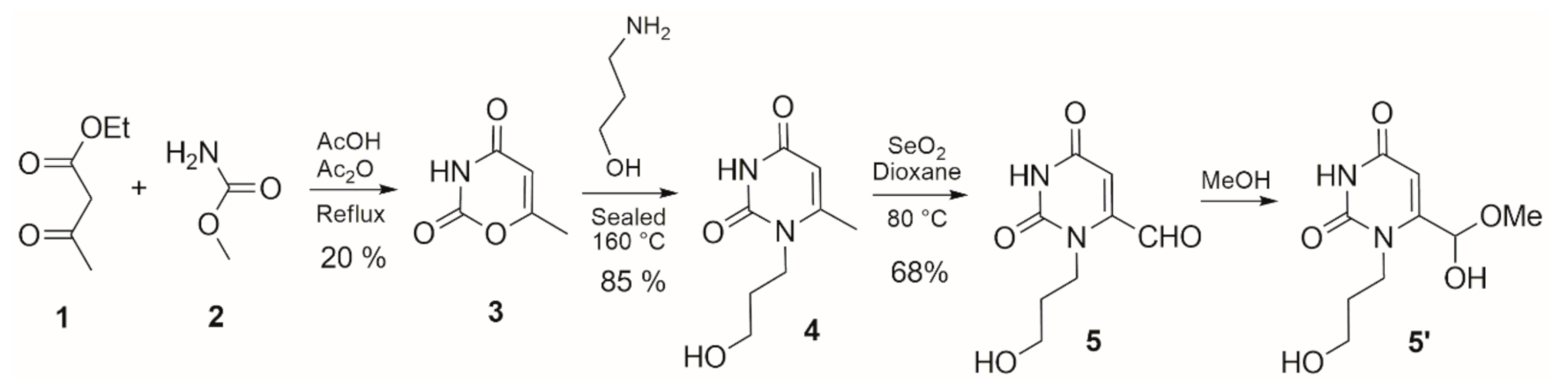
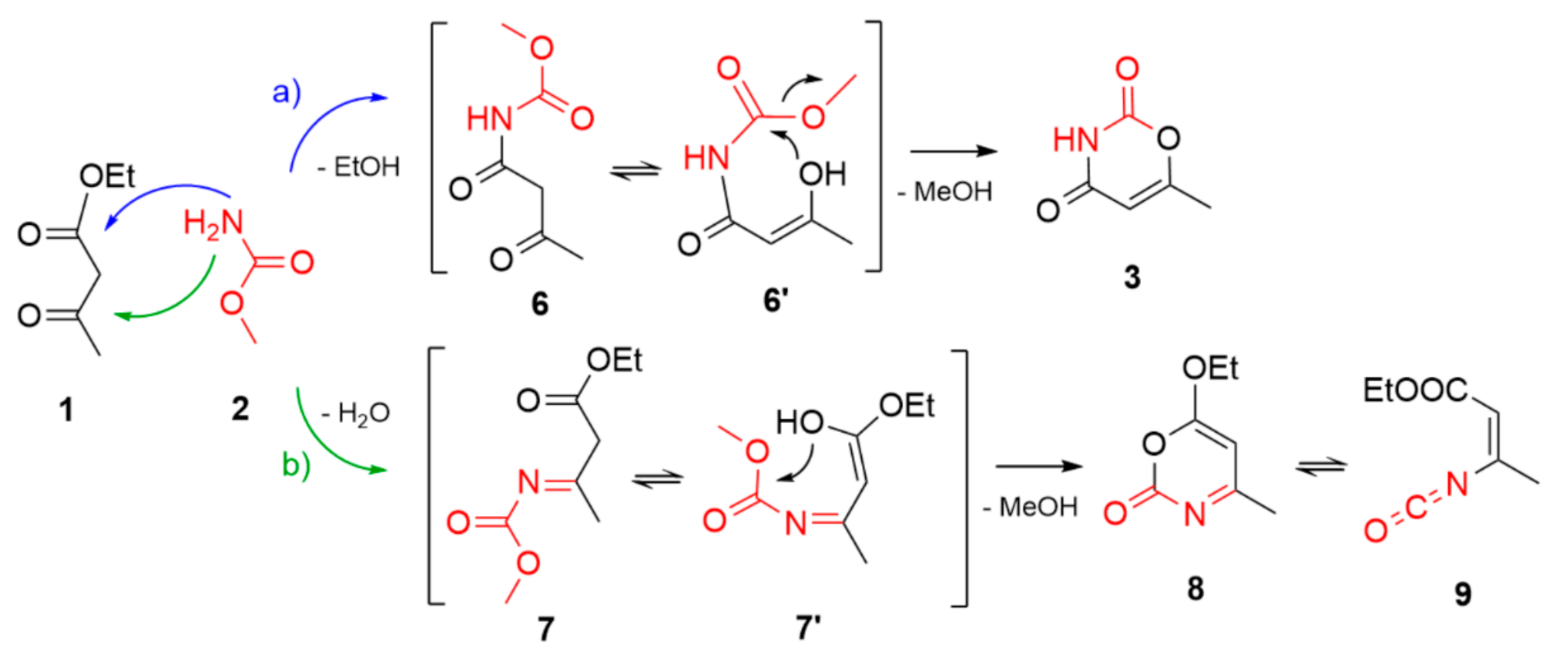
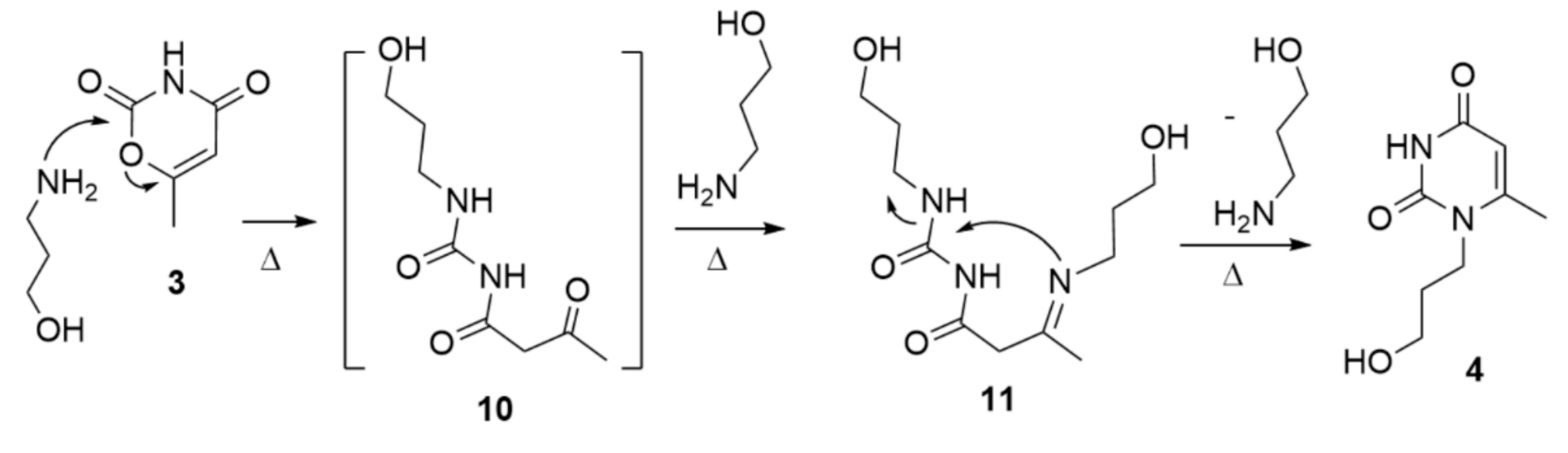
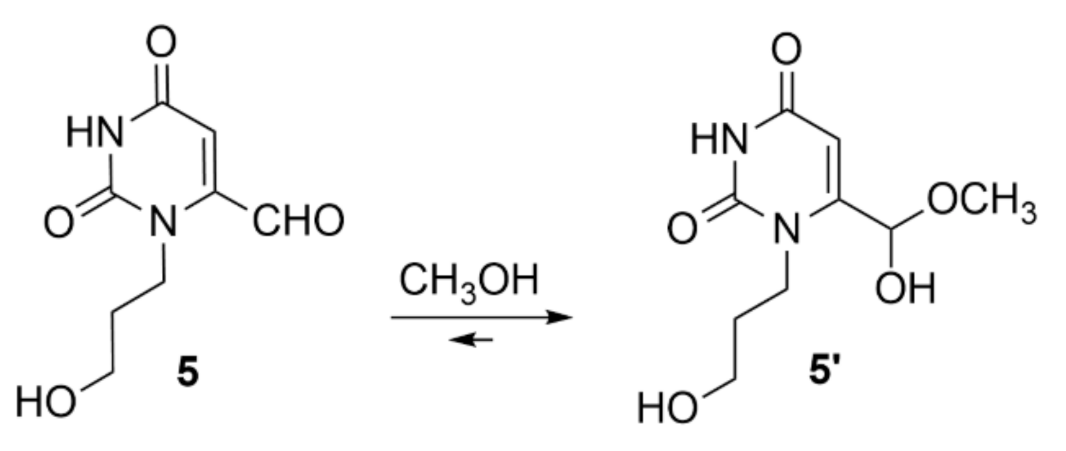
2. Results and Discussion
3. Materials and Methods
3.1. 1-Propanol-6-methyl-uracil 4
3.2. 1-Propanol-6-formyl-uracil Methyl Hemiacetal 5′
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Iha, R.K.; Wooley, K.L.; Nyström, A.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of Orthogonal “Click” Chemistries in the Synthesis of Functional Soft Materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsogoeva, S.B. Recent advances in asymmetric organocatalytic 1,4-conjugate additions. Eur. J. Org. Chem. 2007, 11, 1701–1716. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- LA Ganga, G.; Nardo, V.M.; Cordaro, M.; Natali, M.; Vitale, S.; Licciardello, A.; Nastasi, F.; Campagna, S. A functionalized, ethynyl-decorated, tetracobalt (iii) cubane molecular catalyst for photoinduced water oxidation. Dalton Trans. 2014, 43, 14926–14930. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-Y.; Wang, R.-L.; Xie, L.-J.; Kong, D.-L.; Liu, L.; Cheng, L. A chemical photo-oxidation of 5-Methyl cytidines. Adv. Synth. Catal. 2019, 361, 4685–4690. [Google Scholar] [CrossRef]
- Calabretta, A.; Wasserberg, D.; Posthuma-Trumpie, G.A.; Subramaniam, V.; Van Amerongen, A.; Corradini, R.; Tedeschi, T.; Sforza, S.; Reinhoudt, D.N.; Marchelli, R.; et al. Patterning of peptide nucleic acids using reactive microcontact printing. Langmuir 2011, 27, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Dohno, C.; Shibata, T.; Nakatani, K. Discrimination of N6-methyl adenine in a specific DNA sequence. Chem. Commun. 2010, 46, 5530–5532. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Barkigia, K.M.; Fajer, J.; Drain, C.M. Design and Synthesis of Porphyrins Bearing Rigid Hydrogen Bonding Motifs: Highly Versatile Building Blocks for Self-Assembly of Polymers and Discrete Arrays. J. Org. Chem. 2001, 66, 6513–6522. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.; Elemans, H.; Castriciano, M.A.; Nicosia, A.; Mineo, P.G.; Cordaro, M. A convenient synthetic approach to obtain meso-Uracil-BODIPY. Synlett 2021. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Rohs, R.; West, S.; Sosinsky, A.; Liu, P.; Mann, R.S.; Honig, B. The role of DNA shape in protein–DNA recognition. Nat. Cell Biol. 2009, 461, 1248–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Cao, Z.; Lu, Y. Functional nucleic acid sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, A.; Cordaro, M.; Mazzaglia, A.; Risitano, F.; Venuti, A.; Sciortino, M.T.; Grassi, G. Aldol-type compounds from water-soluble indole-3,4-diones: Synthesis, kinetics, and antiviral properties. Mol. Divers. 2013, 17, 479–488. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Reversible Isomerization in β-Isocyanatocrotonic Esters. Angew. Chem. Int. Ed. 1972, 11, 128–129. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Schwille, D.; Wolf, H. 3-Aza-pyrylium-Salze, VI. Alkyliden-1.3-oxazine und Alkyliden-pyrimidine. Eur. J. Inorg. Chem. 1970, 103, 2760–2767. [Google Scholar] [CrossRef]
- Fresch, E.; Peruffo, N.; Trapani, M.; Cordaro, M.; Bella, G.; Castriciano, M.A.; Collini, E. The effect of hydrogen bonds on the ultrafast relaxation dynamics of a BODIPY dimer. J. Chem. Phys. 2021, 154, 084201. [Google Scholar] [CrossRef]
- Panniello, A.; Trapani, M.; Cordaro, M.; DiBenedetto, C.N.; Tommasi, R.; Ingrosso, C.; Fanizza, E.; Grisorio, R.; Collini, E.; Agostiano, A.; et al. High-Efficiency FRET Processes in BODIPY-Functionalized Quantum Dot Architectures. Chem. Eur. J. 2021, 27, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Mineo, P.; Nastasi, F.; Magazzù, G. Facile synthesis of boronic acids on a BODIPY core with promising sensitivity towards polyols. RSC Adv. 2014, 4, 43931–43933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordaro, M. 3-(3-Hydroxypropyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxaldehyde Methyl Hemiacetal. Molbank 2021, 2021, M1272. https://doi.org/10.3390/M1272
Cordaro M. 3-(3-Hydroxypropyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxaldehyde Methyl Hemiacetal. Molbank. 2021; 2021(3):M1272. https://doi.org/10.3390/M1272
Chicago/Turabian StyleCordaro, Massimiliano. 2021. "3-(3-Hydroxypropyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxaldehyde Methyl Hemiacetal" Molbank 2021, no. 3: M1272. https://doi.org/10.3390/M1272
APA StyleCordaro, M. (2021). 3-(3-Hydroxypropyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxaldehyde Methyl Hemiacetal. Molbank, 2021(3), M1272. https://doi.org/10.3390/M1272






