(±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol
Abstract
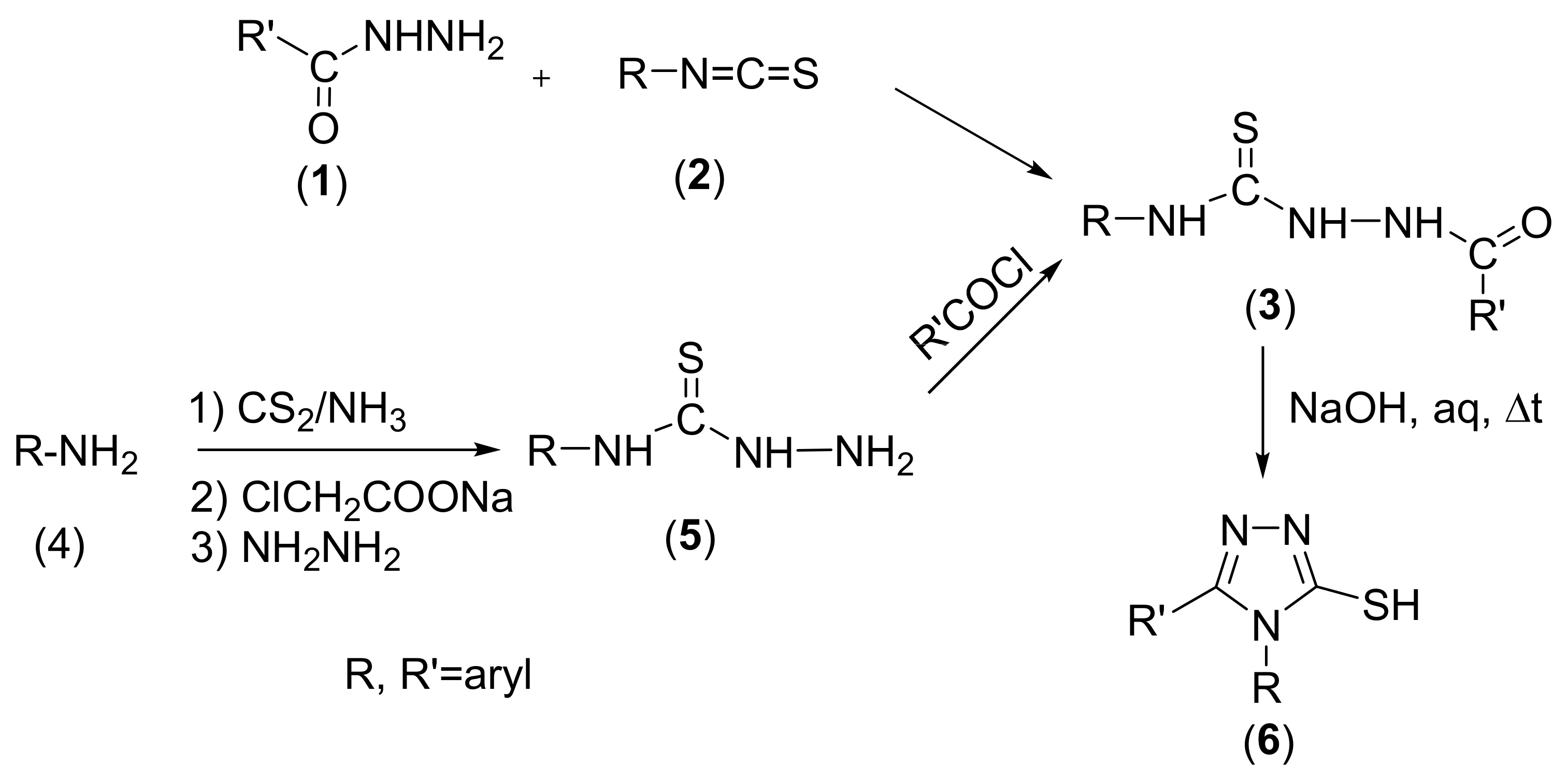
:1. Introduction
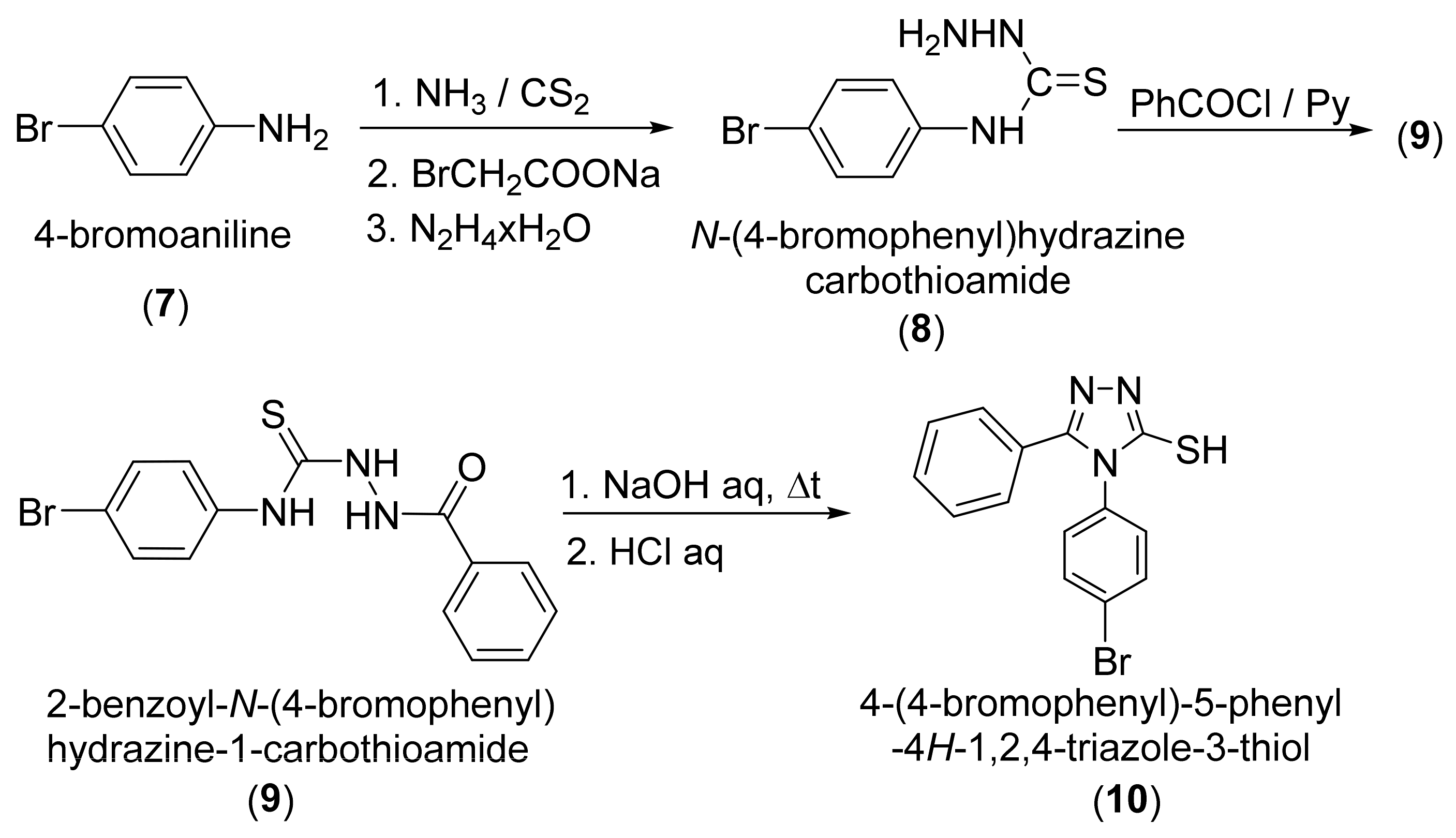
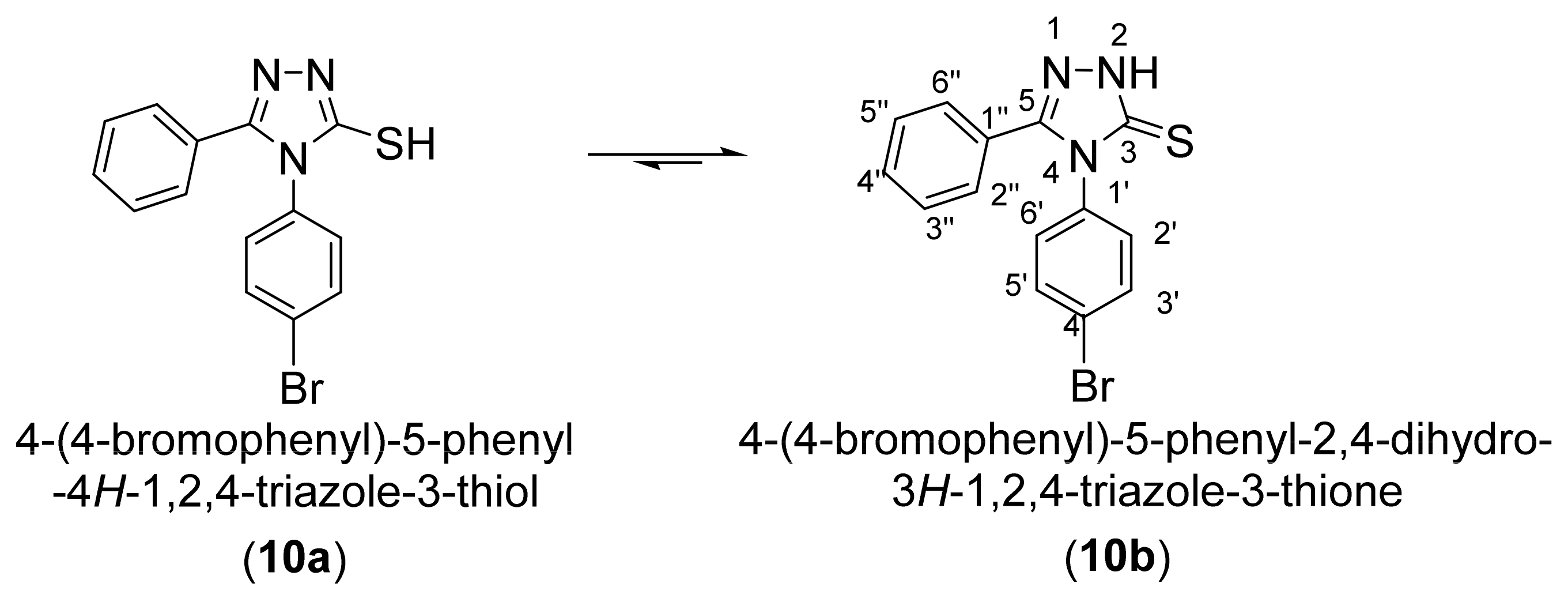
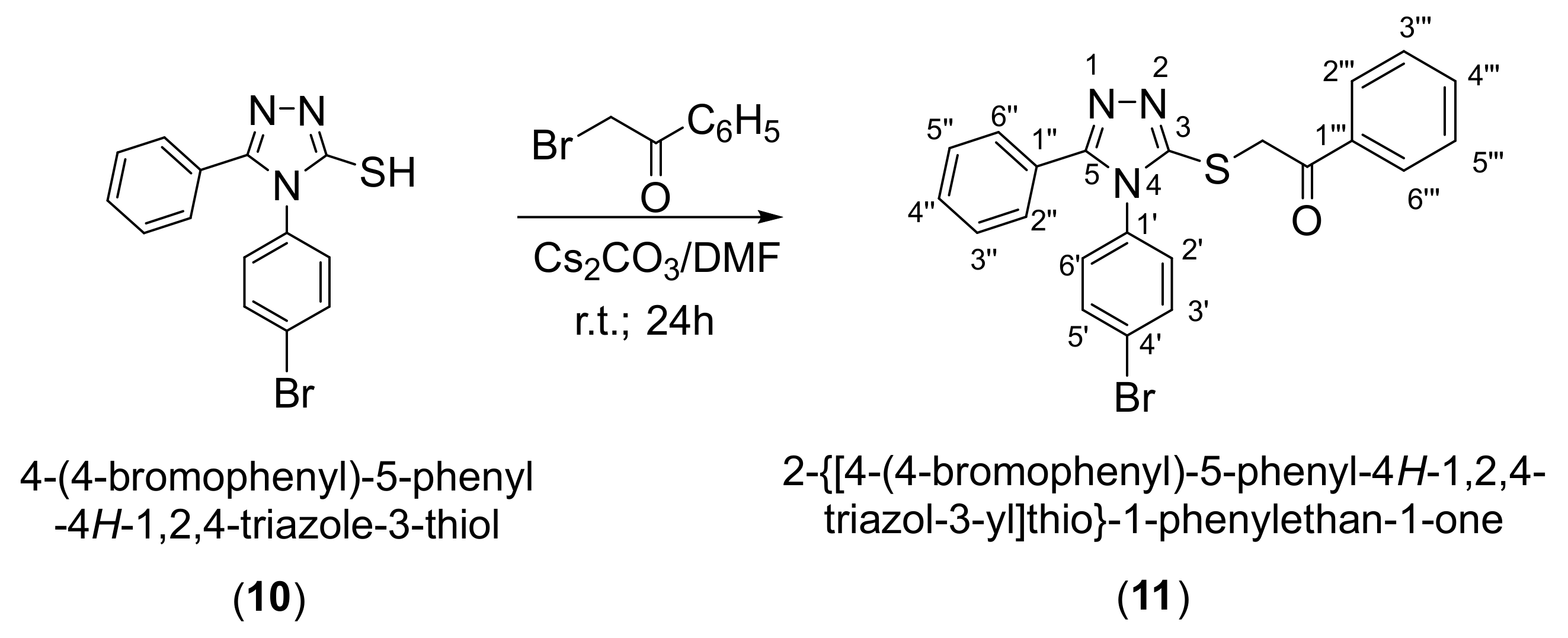
2. Results and Discussion
3. Materials and Methods
3.1. NMR Characterization of 4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazole-3-thiol (10)
3.2. Synthesis of 2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenylethan-1-one (11)
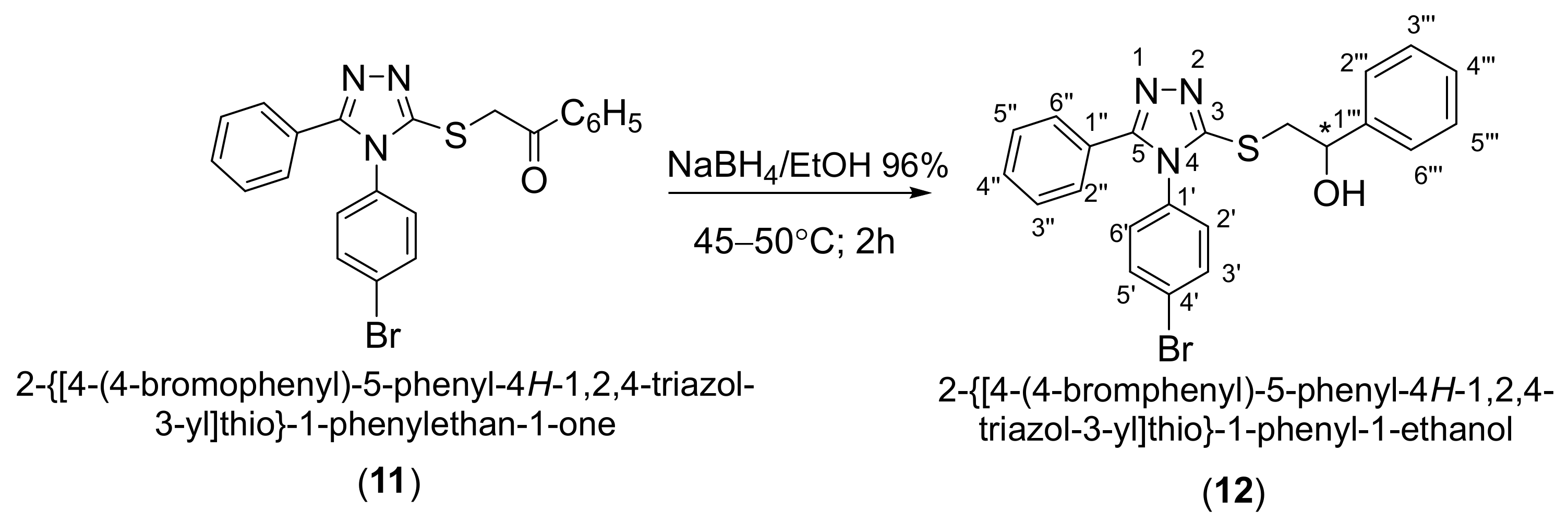
3.3. Synthesis of (±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol (12)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghanaat, J.; Khalilzadeh, M.A.; Zareyee, D. Molecular docking studies, biological evaluation and synthesis of novel 3-mercapto-1,2,4-triazole derivatives. Mol. Divers. 2020, 25, 223–232. [Google Scholar] [CrossRef]
- Plech, T.; Luszczki, J.J.; Wujec, M.; Flieger, J.; Pizoń, M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Karabasanagouda, T.; Adhikari, A.V.; Shetty, N.S. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Behalo, M.S.; Aly, A.A.; Wasfy, A.F.; Rizk, M.M. Synthesis of some novel 1,2,4-triazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2013, 4, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Holla, B.S.; Veerendra, B.; Shivananda, M.K.; Poojary, B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2003, 38, 759–769. [Google Scholar] [CrossRef]
- Duran, A.; Dogan, H.N.; Rollas, S. Synthesis and preliminary anticancer activity of new 1,4-dihydro-3-(3-hydroxy-2-naphthyl)-4-substituted-5H-1,2,4-triazoline-5-thiones. Farmaco 2002, 57, 559–564. [Google Scholar] [CrossRef]
- Kaproń, B.; Czarnomysy, R.; Wysokiński, M.; Andrys, R.; Musilek, K.; Angeli, A.; Supuran, C.T.; Plech, T. 1,2,4-Triazole-based anticonvulsant agents with additional ROS scavenging activity are effective in a model of pharmacoresistant epilepsy. J. Enzym. Inhib. Med. Chem. 2020, 35, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent advances bioactive 1,2,4-triazole-3-thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef]
- Al-Aabdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-Inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Drug Des. Dev. Ther. 2014, 8, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Godhani, D.R.; Sanja, D.B.; Sanghani, A.M. Synthesis and antimicrobial elucidation of [1,2,4]-triazole-3-thione derivatives. J. Chem. Pharm. Res. 2013, 5, 240–243. [Google Scholar]
- Goswami, B.N.; Kataky, J.C.S.; Baruah, J.N. Synthesis and antibacterial activity of 1-(2,4-dichlorobenzoyl)-4-substituted thiosemicarbazides, 1,2,4-triazoles and their methyl derivatives. J. Heterocycl. Chem. 1984, 21, 1225–1229. [Google Scholar] [CrossRef]
- Tehranchian, S.; Akbarzadeh, T.; Fazeli, M.; Reza, M.; Jamalifar, H.; Shafiee, A. Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorg. Med. Chem. Lett. 2005, 15, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sul-fonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Zołnierek, M.; Nowak, G. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on GABAergic system. Eur. J. Med. Chem. 2014, 86, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Nuțiu, M.; Bercean, V.; Birău, M. Synthesis of some 4-aryl-thiosemicarbazides. Ann. West Univ. Timişoara 1996, 5, 7–10. [Google Scholar]
- Maxwell, J.R.; Wasdahl, D.A.; Wolfson, A.C.; Stenberg, V.I. Synthesis of 5-aryl-2H-tetrazoles, 5-Aryl-2H-tetrazole-2-acetic acids, and [(4-phenyl-5-aryl-4H-1,2,4-triazol-3-yl)thio]acetic acids as possible superoxide scavengers and antiinflammatory agents. J. Med. Chem. 1984, 27, 1565–1570. [Google Scholar] [CrossRef]
- Wang, L.-C.; Tang, J.; Wei, T.-B.; Zhang, Y.-M. Synthesis and biological bctivity of 2-(3-phenoxymethyl-4-phenyl-[1,2,4]triazole-5-thio)acetic acid. Chin. J. Org. Chem. 2008, 28, 343–347. [Google Scholar] [CrossRef]
- Dimri, A.K.; Parmar, S.S. Synthesis of 3-aryl-4-oxothiazolin-2-yl(4-ethoxy-3-methoxy)phenyl hydrazones as possible anti-convulsants. J. Heterocycl. Chem. 1978, 15, 335–336. [Google Scholar] [CrossRef]
- Radl, S. Preparation of some pyrazole derivatives by extrusion of elemental sulfur from 1,3,4-thiadiazines. J. Heterocycl. Chem. 1992, 57, 656–659. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Behyar, T. Fast and efficient method for reduction of carbonyl compounds with NaBH4 /wet SiO2 under solvent free condition. J. Braz. Chem. Soc. 2005, 16, 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Toda, F.; Kiyoshige, K.; Yagi, M. NaBH4 Reduction of Ketones in the Solid State. Angew. Chem. Int. Ed. Engl. 1989, 28, 320–321. [Google Scholar] [CrossRef]
- Fizer, M.; Slivka, M.; Korol, N.; Fizer, O. Identifying and explaining the regioselectivity of alkylation of 1,2,4-triazole-3-thiones using NMR, GIAO and DFT methods. J. Mol. Struct. 2021, 1223, 128973. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorga, M.M.; Badea, V. (±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol. Molbank 2021, 2021, M1268. https://doi.org/10.3390/M1268
Vorga MM, Badea V. (±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol. Molbank. 2021; 2021(3):M1268. https://doi.org/10.3390/M1268
Chicago/Turabian StyleVorga, Milenca Mariana, and Valentin Badea. 2021. "(±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol" Molbank 2021, no. 3: M1268. https://doi.org/10.3390/M1268
APA StyleVorga, M. M., & Badea, V. (2021). (±)-2-{[4-(4-Bromophenyl)-5-phenyl-4H-1,2,4-triazol-3-yl]sulfanyl}-1-phenyl-1-ethanol. Molbank, 2021(3), M1268. https://doi.org/10.3390/M1268





