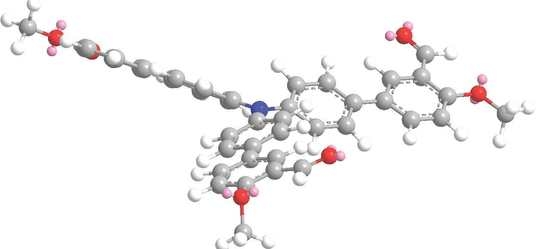
4′,4′′′,4′′′′′-Nitrilotris(4-methoxy-[1,1′-biphenyl]-3-carbaldehyde)
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Consideration
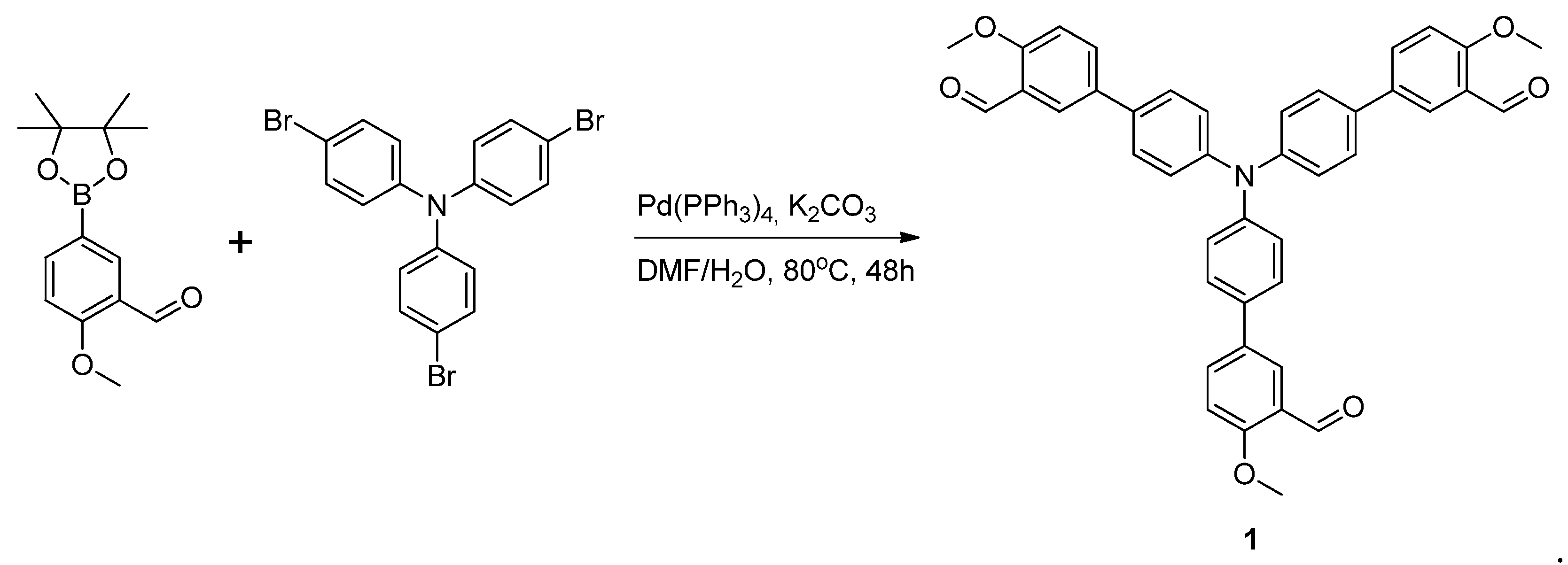
3.2. Synthesis of 4′,4′′′,4′′′′′-Nitrilotris(4-Methoxy-[1,1′-biphenyl]-3-carbaldehyde)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kretz, T.; Bats, J.W.; Lerner, H.-W.; Wagner, M. 2,5-Diformylbenzene-1,4-diol: A Versatile Building Block for the Synthesis of Ditopic Redox-Active Schiff Base Ligands. Z. Nat. B 2007, 62, 66–74. [Google Scholar] [CrossRef]
- Grudzień, K.; Malinska, M.; Barbasiewicz, M. Synthesis and Properties of Bimetallic Hoveyda–Grubbs Metathesis Catalysts. Organometallics 2012, 31, 3636–3646. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Liu, Y.; Han, H.; Li, Z.; Chu, W.; Sun, Z. Efficient symmetrical bidentate dioxime ligand-accelerated homogeneous palladium-catalyzed Suzuki–Miyaura coupling reactions of aryl chlorides. New J. Chem. 2016, 41, 372–376. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Lukyanov, D.A.; Vlasov, P.S.; Yankin, A.N.; Atangulov, A.B.; Sizov, V.V.; Levin, O.V. Nickel Salicylaldoxime-Based Coordination Polymer as a Cathode for Lithium-Ion Batteries. Energies 2020, 13, 2480. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Talati, A.M. Organometallic Semiconductors. I. Derivatives of Oximes of 1,5-Diacyl-2,6-dihydroxynaphthalenes. J. Am. Chem. Soc. 1964, 86, 1592–1595. [Google Scholar] [CrossRef]
- Manecke, G.; Wille, W.E.; Kossmehl, G. Preparation and properties of monomeric and polymeric Schiff bases derived from salicylaldehyde and 2,5-dihydroxyterephthalaldehyde. II. Electrical conductivity. Makromol. Chem. 1972, 160, 111–126. [Google Scholar] [CrossRef]
- Okada, Y.; Sugai, M.; Chiba, K. Hydrogen-Bonding-Induced Fluorescence: Water-Soluble and Polarity-Independent Solvatochromic Fluorophores. J. Org. Chem. 2016, 81, 10922–10929. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, U.S. Liquid crystalline bis(N-salicylideneaniline)s: Synthesis and thermal behavior of constitutional isomers. Tetrahedron Lett. 2013, 54, 3419–3423. [Google Scholar] [CrossRef]
- Huang, J.; Han, X.; Yang, S.; Cao, Y.; Yuan, C.; Liu, Y.; Wang, J.-G.; Cui, Y. Microporous 3D Covalent Organic Frameworks for Liquid Chromatographic Separation of Xylene Isomers and Ethylbenzene. J. Am. Chem. Soc. 2019, 141, 8996–9003. [Google Scholar] [CrossRef]
- Mastalerz, M.; Oppel, I.M. Synthesis of Tetrahedral Shape-Persistent Tetranuclear Metal-salphens. Eur. J. Org.Chem. 2011, 2011, 5971–5980. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, A.A.; Novoselova, J.V.; Kalnin, A.Y.; Lukyanov, D.A.; Levin, O.V. 4′,4′′′,4′′′′′-Nitrilotris(4-methoxy-[1,1′-biphenyl]-3-carbaldehyde). Molbank 2021, 2021, M1263. https://doi.org/10.3390/M1263
Vereshchagin AA, Novoselova JV, Kalnin AY, Lukyanov DA, Levin OV. 4′,4′′′,4′′′′′-Nitrilotris(4-methoxy-[1,1′-biphenyl]-3-carbaldehyde). Molbank. 2021; 2021(3):M1263. https://doi.org/10.3390/M1263
Chicago/Turabian StyleVereshchagin, Anatoliy A., Julia V. Novoselova, Arseniy Y. Kalnin, Daniil A. Lukyanov, and Oleg V. Levin. 2021. "4′,4′′′,4′′′′′-Nitrilotris(4-methoxy-[1,1′-biphenyl]-3-carbaldehyde)" Molbank 2021, no. 3: M1263. https://doi.org/10.3390/M1263
APA StyleVereshchagin, A. A., Novoselova, J. V., Kalnin, A. Y., Lukyanov, D. A., & Levin, O. V. (2021). 4′,4′′′,4′′′′′-Nitrilotris(4-methoxy-[1,1′-biphenyl]-3-carbaldehyde). Molbank, 2021(3), M1263. https://doi.org/10.3390/M1263