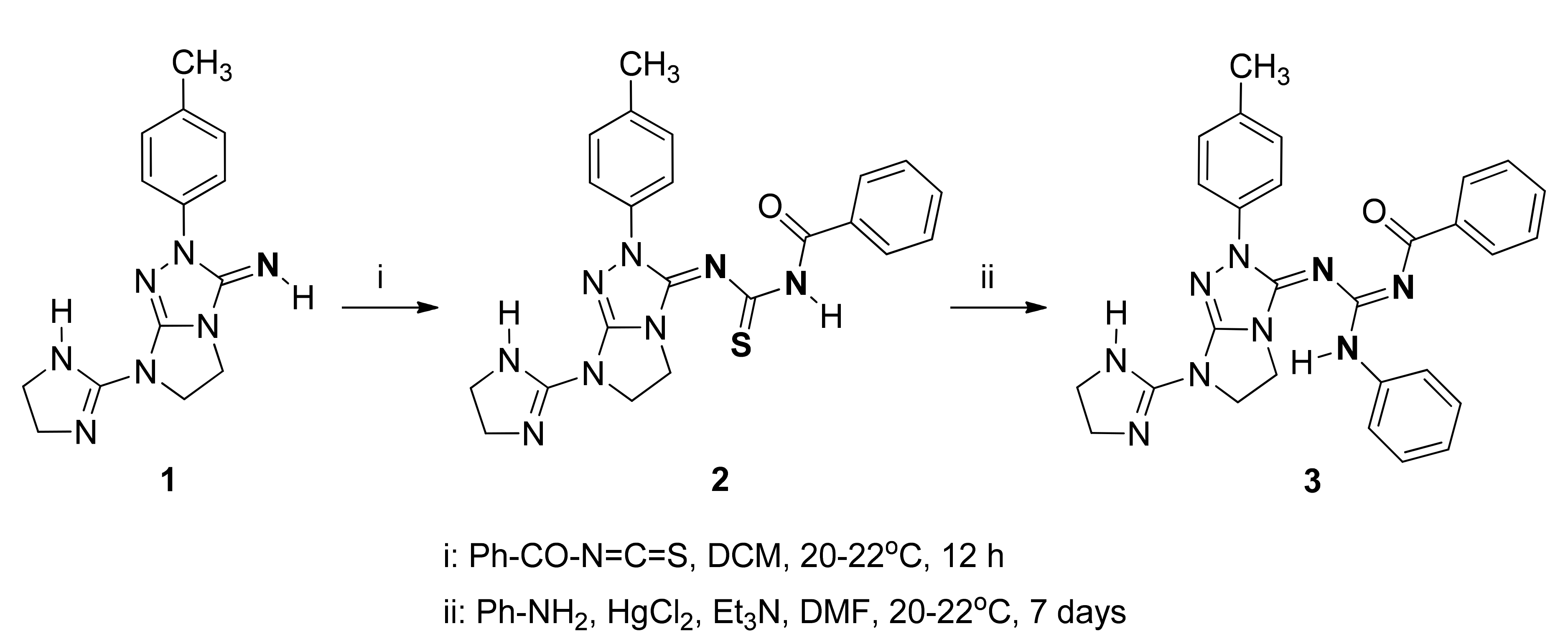
Synthesis of the Guanidine Derivative: N-{[(7-(4,5-Dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene)amino](phenylamino)methylene}benzamide
Abstract
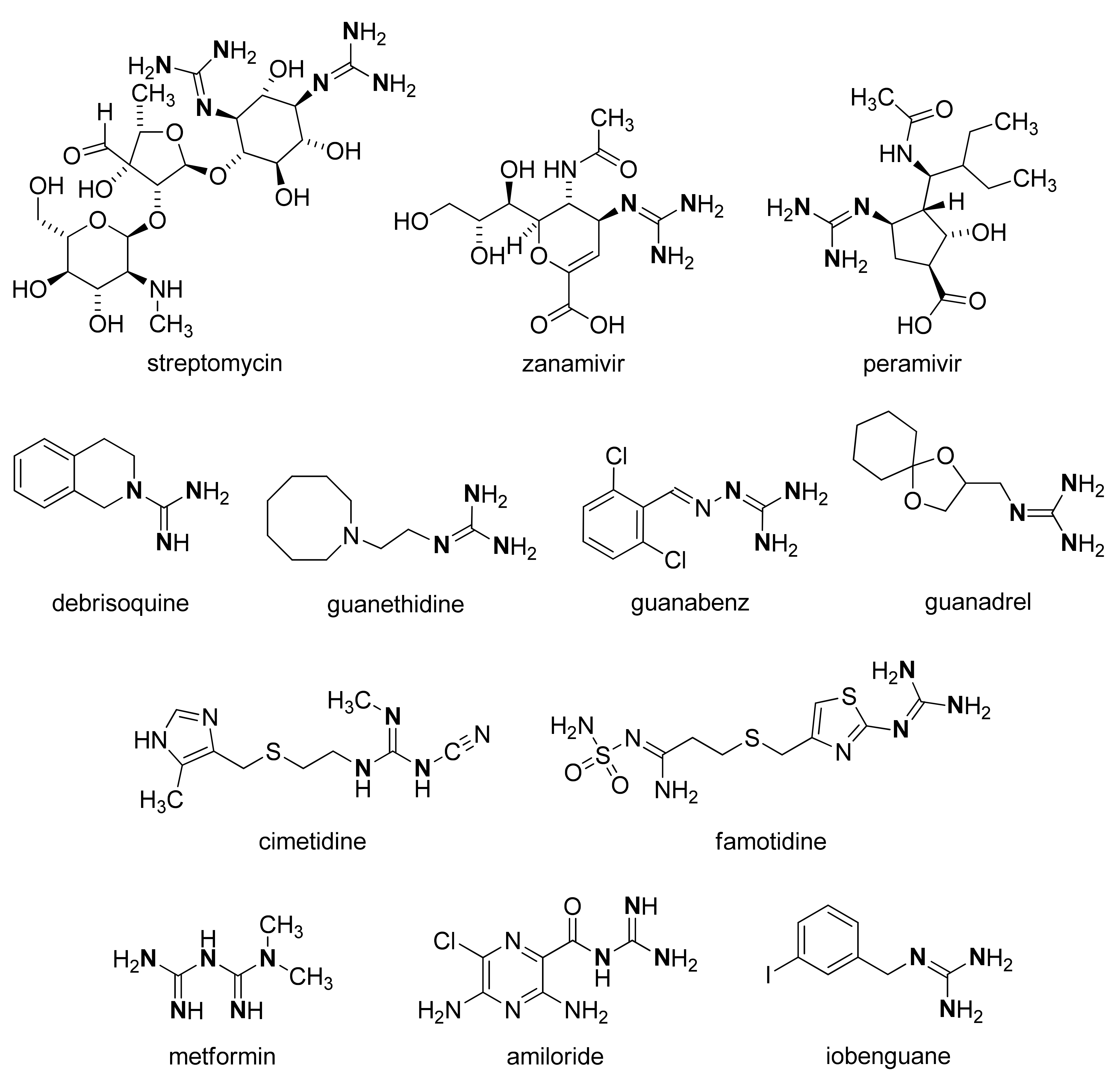
:1. Introduction
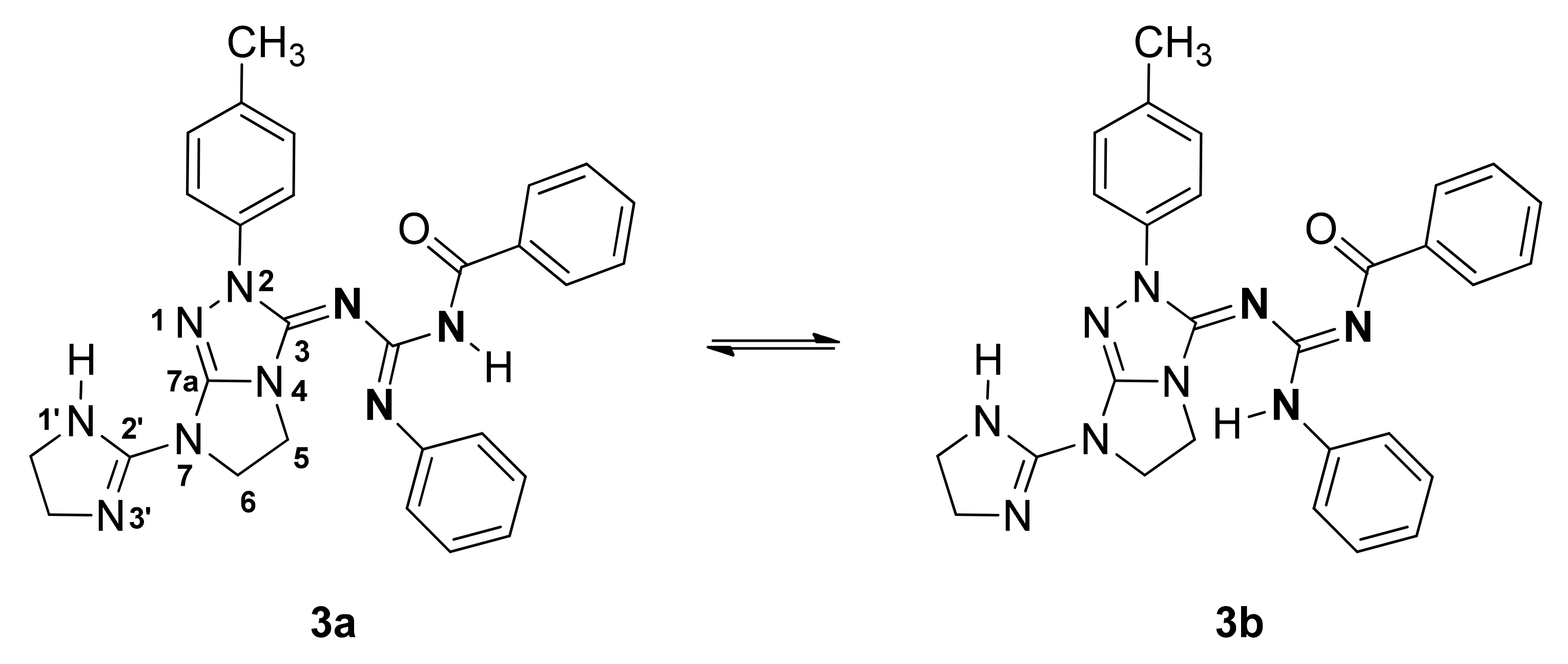
2. Results and Discussion
3. Materials and Methods
3.1. General Methods and Physical Measurements
3.2. Synthesis of N-{[7-(4,5-Dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene]carbamothioyl}benzamide (2)
3.3. Synthesis of N-{[(7-(4,5-dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene)amino](phenylamino)methylene}benzamide (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berlinck, R.G.S.; Romminger, S. The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 2016, 33, 456–490. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ciavatta, M.L.; Mathieu, V.; Ingels, A.; Kiss, R.; Pascale, P.; Mollo, E.; Ungur, N.; Guo, Y.-W.; Gavagnin, M. Marine terpenoid diacylguanidines: Structure, synthesis, and biological evaluation of naturally occurring actinofide and synthetic analogues. J. Nat. Prod. 2017, 80, 1339–1346. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Pegan, S.D.; Franzblau, S.G.; Orjala, J. An antimicrobial guanidine bearing sesterterpene from cultured cyanobacterium Scytonema sp. J. Nat. Prod. 2009, 72, 2043–2045. [Google Scholar] [CrossRef] [Green Version]
- Pryma, D.; Chin, B.; Noto, R.; Dillon, J.; Solnes, L.; White, T.; Stambler, N.; Lin, T.; DiPippo, V.A.; Jensen, J.D.; et al. Azedra (iobenguane I 131) in patients with malignant, recurrent and/or unresectable pheochromocytoma or paraganglioma (PPGL): Updated efficacy and safety results from a multi-center, open-label, pivotal phase 2 study. J. Clin. Oncol. 2018, 36, 4005. [Google Scholar] [CrossRef]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and evaluation of anticancer activities of novel C-28 guanidine-functionalized triterpene acid derivatives. Molecules 2018, 23, 3000. [Google Scholar] [CrossRef] [Green Version]
- Duca, G.; Aricu, A.; Kuchkova, K.; Secara, E.; Barba, A.; Dragalin, I.; Ungur, N.; Spengler, G. Synthesis, structural elucidation and biological evaluations of new guanidine-containing terpenoids as anticancer agents. Nat. Prod. Res. 2019, 33, 3052–3056. [Google Scholar] [CrossRef]
- Zarraga, M.; Zarraga, A.M.; Rodriguez, B.; Perez, C.; Paz, C.; Paz, P.; Sanhueza, C. Synthesis of a new nitrogenated drimane derivative with antifungal activity. Tetrahedron Lett. 2008, 49, 4775–4776. [Google Scholar] [CrossRef]
- Sidoryk, K.; Świtalska, M.; Rózga, P.; Wietrzyk, J.; Bujak, I.; Żerek, B.; Kaczmarek, Ł.; Cybulski, M. An efficient synthesis of indolo[2,3-b]quinoline guanidine derivatives with their in vitro and in vivo study. Med. Chem. Res. 2017, 26, 3354–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohara, K.; Smietana, M.; Restouin, A.; Mollard, S.; Borg, J.-P.; Collette, Y.; Vasseur, J.-J. Amine-guanidine switch: A promising approach to improve DNA binding and antiproliferative activities. Med. Chem. 2007, 50, 6465–6475. [Google Scholar] [CrossRef] [PubMed]
- Previtali, V.; Trujillo, C.; Amet, R.; Zisterer, D.M.; Rozas, I. Effect of isouronium/guanidinium substitution on the efficacy of a series of novel anti-cancer agents. Med. Chem. Commun. 2018, 9, 735–743. [Google Scholar] [CrossRef]
- Schmidhammer, H.; Spetea, M.; Windisch, P.; Schütz, J.; Riba, P.; Al-Khrasani, M.; Fürst, S. Functionalization of the carbonyl group in position 6 of morphinan-6-ones. Development of novel 6-amino and 6-guanidino substituted 14 alkoxymorphinans. Curr. Pharm. Des. 2013, 19, 7391–7399. [Google Scholar] [CrossRef] [PubMed]
- Gund, P. Guanidine, trimethylenemethane, and “Y-delocalization”. Can acyclic compounds have “aromatic” stability? J. Chem. Educ. 1972, 49, 100–103. [Google Scholar] [CrossRef]
- Reetz, M.T.; Bingel, C.; Harms, K. Structure of carbanions having carbocations as counterions. J. Chem. Soc. Chem. Commun. 1993, 1558–1560. [Google Scholar] [CrossRef]
- Binoy, J.; James, C.; Joe, I.H.; Jayakumar, V.S. Vibrational analysis and Y-aromaticity in bis(N,N’-diphenylguanidinium) oxalate crystal: A DFT study. J. Mol. Struct. 2006, 784, 32–46. [Google Scholar] [CrossRef]
- Balewski, Ł.; Sączewski, F.; Bednarski, P.J.; Wolff, L.; Nadworska, A.; Gdaniec, M.; Kornicka, A. Synthesis, structure and cytotoxicity testing of novel 7-(4,5-dihydro-1H-imidazol-2-yl)-2-aryl-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-imine derivatives. Molecules 2020, 25, 5924. [Google Scholar] [CrossRef] [PubMed]
- Maryanoff, J.C.; Stanzione, R.C.; Plampin, J.N.; Mills, J.E. A convenient synthesis of guanidines from thioureas. J. Org. Chem. 1986, 51, 1882–1884. [Google Scholar] [CrossRef]
- Kim, K.S.; Qian, L. Improved method for the preparation of guanidines. Tetrahedron Lett. 1993, 34, 7677–7680. [Google Scholar] [CrossRef]
- Sączewski, F.; Kornicka, A.; Rybczyńska, A.; Hudson, A.L.; Miao, S.S.; Gdaniec, M.; Boblewski, K.; Lehmann, A. 1-[(Imidazolidin-2-yl)imino]indazole. Highly α2/I1 selective agonist: Synthesis, X-ray structure, and biological activity. J. Med. Chem. 2008, 51, 3599–3608. [Google Scholar] [CrossRef]
- McMullan, M.; Kelly, B.; Mihigo, H.B.; Keogh, A.P.; Rodriguez, F.; Brocos-Mosquera, I.; García-Bea, A.; Miranda-Azpiazu, P.; Callado, L.F.; Rozas, I. Di-aryl guanidinium derivatives: Towards improved α2-adrenergic affinity and antagonist activity. Eur. J. Med. Chem. 2021, 209, 112947. [Google Scholar] [CrossRef]
- Shibanuma, T.; Shiono, M.; Mukaiyama, T. A convenient method for the preparation of carbodiimides using 2-chloropyridinium salt. Chem. Lett. 1977, 6, 575–576. [Google Scholar] [CrossRef] [Green Version]
- Yong, Y.F.; Kowalski, J.A.; Lipton, M.A. Facile and efficient guanylation of amines using thioureas and Mukaiyama’s Reagent. J. Org. Chem. 1997, 62, 1540–1542. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rogovoy, B.V. Recent developments in guanylating agents. ARKIVOC 2005, IV, 49–87. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balewski, Ł.; Kornicka, A. Synthesis of the Guanidine Derivative: N-{[(7-(4,5-Dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene)amino](phenylamino)methylene}benzamide. Molbank 2021, 2021, M1246. https://doi.org/10.3390/M1246
Balewski Ł, Kornicka A. Synthesis of the Guanidine Derivative: N-{[(7-(4,5-Dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene)amino](phenylamino)methylene}benzamide. Molbank. 2021; 2021(3):M1246. https://doi.org/10.3390/M1246
Chicago/Turabian StyleBalewski, Łukasz, and Anita Kornicka. 2021. "Synthesis of the Guanidine Derivative: N-{[(7-(4,5-Dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene)amino](phenylamino)methylene}benzamide" Molbank 2021, no. 3: M1246. https://doi.org/10.3390/M1246
APA StyleBalewski, Ł., & Kornicka, A. (2021). Synthesis of the Guanidine Derivative: N-{[(7-(4,5-Dihydro-1H-imidazol-2-yl)-2-(p-tolyl)-6,7-dihydro-2H-imidazo[2,1-c][1,2,4]triazol-3(5H)-ylidene)amino](phenylamino)methylene}benzamide. Molbank, 2021(3), M1246. https://doi.org/10.3390/M1246







