Abstract
The 1,2,3-triazole is a well-known biologically active pharmacophore constructed by the copper-catalyzed azide–alkyne cycloaddition. We herein reported the synthesis of 4-amino-7-chloro-based [1,2,3]-triazole hybrids via Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of 4-azido-7-chloroquinoline with an alkyne derivative of acetaminophen. The compound was fully characterized by Fourier-transform infrared (FTIR), proton nuclear magnetic resonance (1H-NMR), carbon-13 nuclear magnetic resonance (13C-NMR), heteronuclear single quantum coherence (HSQC), ultraviolet (UV) and high-resolution mass spectroscopies (HRMS). This compound was screened in vitro with different normal and cancer cell lines. The drug likeness of the compound was also investigated by predicting its pharmacokinetic properties.
1. Introduction
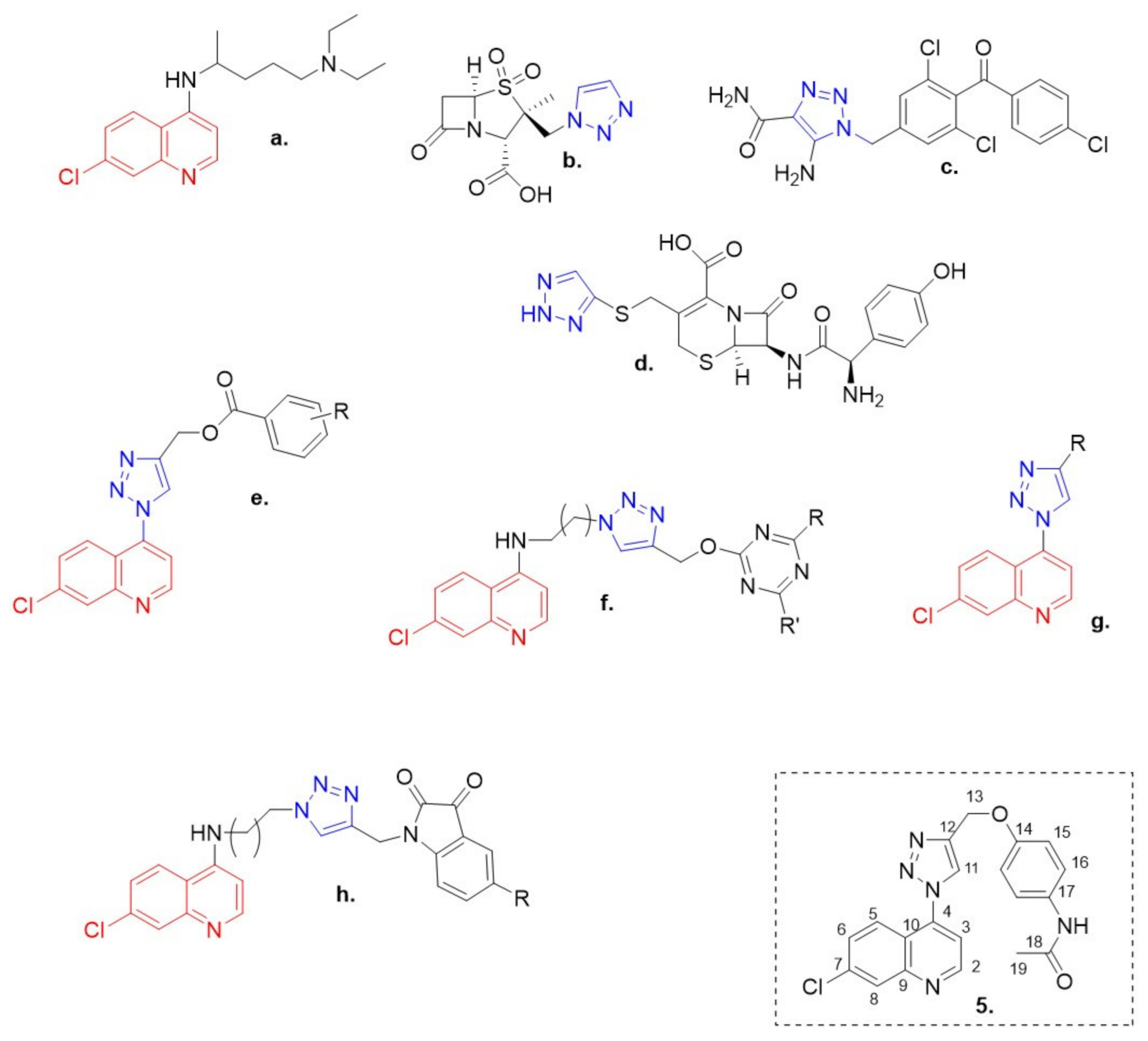
The 7-chloroquinoline moiety, a pharmacophore of several established antimalarial drugs such as chloroquine (Figure 1a) [1], is recently being focused on as a potential anti-cancer agent as well as a chemosensitizer when used in combination with anti-cancer drugs [2].
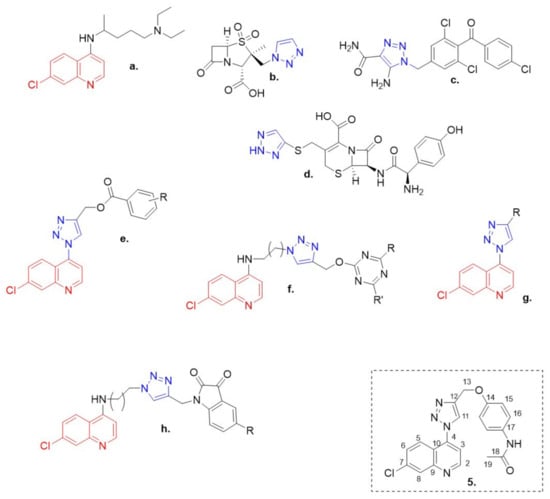
Figure 1.
Clinically approved quinoline (red) and 1,2,3-triazole (blue) containing drugs. (a) Chloroquine; (b) tazobactam; (c) carbonic anhydrase inhibitors (CAI); (d) cefatrizine; (e–h) some structures of quinolone- and triazole-based hybrids reported in literature and compound 5 reported in this paper.
With increasing drug resistance to available agents, intensive drug discovery efforts on developing new antimalarial/anticancer drugs or modifying existing agents are ongoing [3]. Molecular hybridization as a drug discovery strategy involves the rational design of new chemical entities by the fusion (usually via a covalent linker) of two drugs, of which both active compounds and/or pharmacophoric units are recognized and derived from known bioactive molecules [3].
In order to broaden the structural diversity of the compounds and intensify their biological activities, covalently linked hybrids were created with a pharmacologically significant class of compounds known as [1,2,3]-triazole (Figure 1b–d). These [1,2,3]-triazoles have exhibited a myriad of biological activities including antifungal, [4] antibacterial [5] and antitubercular activities [6]. Some structures of quinolone- and triazole-based hybrids reported in literature [7,8,9,10] (Figure 1e–h).
We have previously reported the conversion of the commercially available 4,7-dichloroquinoline 1 to a series of 4-amino-7-chloroquinolone derivatives [11]. Herein, we reported the synthesis of a novel 4-amino-7-chloroquinoline-based 1,2,3-triazole hybrid 5 by Cu(I)-catalyzed azide–alkyne cycloaddition. The structure of compound 5 was characterized by NMR, MS, FT-IR and UV spectra. The cytotoxicity of 5 was also evaluated against different cell lines.
2. Results and Discussion
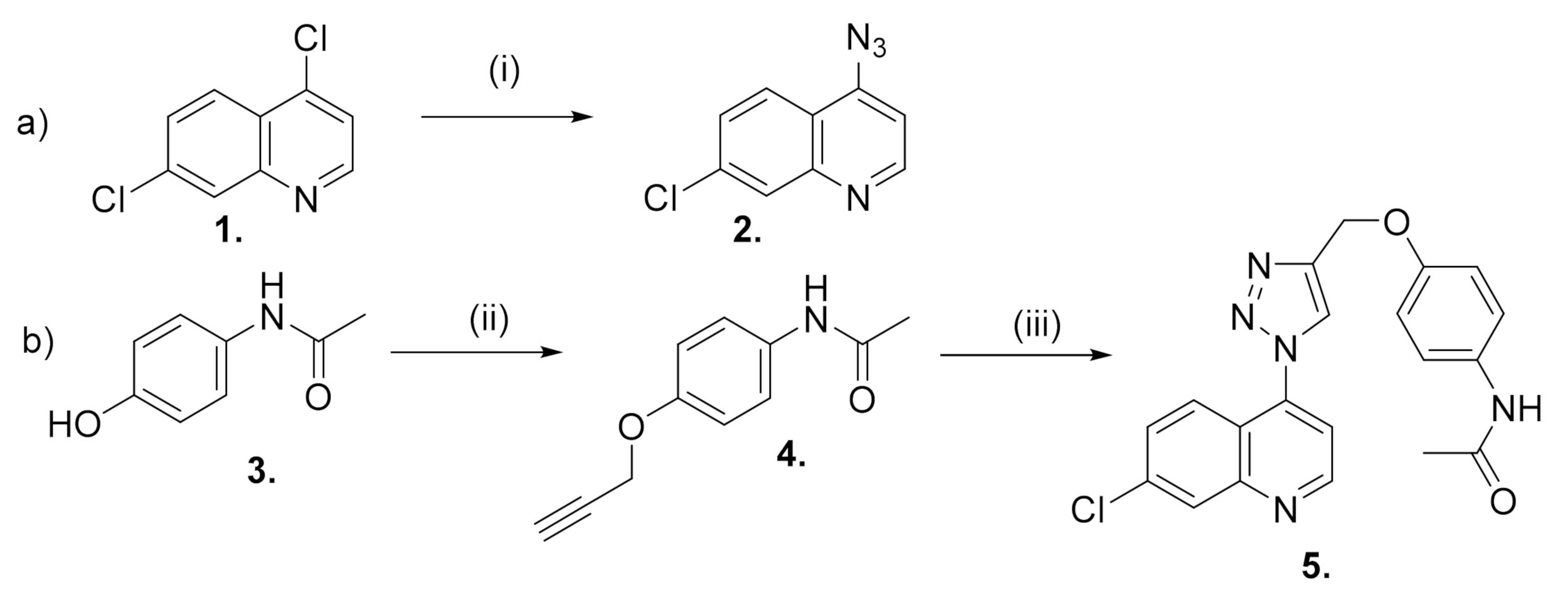
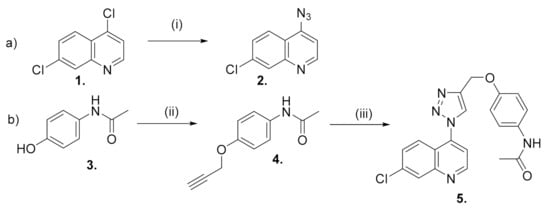
The synthesis of the triazole hybrid compound, N-[7-chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl] quinoline]-acetamide 5, involved the initial synthesis of the precursors 4-azido-7-chloroquinoline 2 and O-acetylenic acetaminophen 4.
Quinoline 2 was furnished by applying the modified method reported by de Souza et al. [12], whereby 4,7-dichloroquinoline 1 reacted with two equivalents of NaN3 in anhydrous DMF at 65 °C for 6 h (Scheme 1a). The recrystallization of the crude product from CH2Cl2/hexane afforded 2 in an 86% yield.
Scheme 1.
(a) Synthesis of 4-azido-7-chloro-quinoline. (i) NaN3, DMF; (b) Synthesis of N-[7-chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide. (ii) propargyl bromide, DMF, K2CO3; (iii) 2, CuSO4, ascorbic acid, tBuOH/water (1:1).
The O-alkylation reaction of acetaminophen 3 with 1.5 equivalents of propargyl bromide and anhydrous K2CO3 in anhydrous DMF yielded the acetylenic intermediate 4 in a good yield after the recrystallization from CH2Cl2/hexane (82%, Scheme 1b).
The hybrid compound 5 was finally obtained by using a modified cycloaddition procedure reported by Fokin et al. [13].
Equimolars of quinoline 2 and acetylene 4 were dissolved in tBuOH/water (1:1) and were treated with sodium ascorbate (0.4 equiv.) and CuSO4 (20 mol%) sequentially (Scheme 1b). The reaction mixture was then stirred vigorously at 65 °C for 24 h. Compound 5 was isolated by column chromatography in a high yield (72%).
The structure of 5 was verified by 1H and 13C NMR spectra (Supplementary Materials, Figures S3 and S4). The 1H-NMR spectrum showed a singlet at 5.33 ppm associated with the C13-methylene group and a singlet at 2.07 ppm associated with the α-position of the C18-carbonyl group.
Regarding the 13C NMR signals, the disappearance of characteristic peaks of acetylenic group at 78.5 and 75.5 ppm, and the appearance of a C-13 methylene signal at 61.5 ppm and C-11 vinylic signal at 127.3 ppm were probably the most relevant features to verify the incorporation of a triazole moiety.
Heteronuclear single quantum coherence spectroscopy (HSQC) was used to assign 13C signals of compound 5 as shown in Table S1 (see Supplementary Materials for 2D spectra, Figures S5 and S6).
The IR spectrum of compound 5 showed characteristic N–H stretching at 3448 cm−1, C=O stretching at 1674 cm−1 and C=C stretching at 1612 cm−1 (Supplementary Materials, Figure S7). Other vibrational peaks at 1550, 1512 and 1411 cm−1 were corresponding to the N–H bending in the amide. Moreover, the absence of the azide stretching peak of 2 at 2123 cm−1 in the IR spectrum of 5 confirmed the conversion of azide.
The UV and HRMS of 5 was also recorded for further characterization (Supplementary Materials, Figures S8 and S9).
The target compound 5 showed a low cytotoxicity by evaluating its in vitro cytotoxic activities against normal (LO2 and BEAS-2B), immortalized (HEK293) and cancer (HepG2 and A549) cell lines (IC50 values > 100 μM, Supplementary Materials Figure S10).
In addition, the promising compound 5 was assessed by predicting its physicochemical properties and oral bioavailability. From the calculated physicochemical properties (Supplementary Materials Table S2), compound 5 did not violate any of Lipinski’s rules [14], indicating its drug-like character and a good chance for oral administration.
This finding corroborates the results of the gastrointestinal absorption from SwissADME, in which compound 5 was predicted with high absorption according to BOILED-Egg model [15] (Figure S11) and data from admetSAR 2 [16], in which the compound was predicted to be orally bioavailable and absorbed in human intestine.
3. Materials and Methods
3.1. Chemistry
Silica gel (FCP 230–400 mesh) was used for column chromatography. Thin-layer chromatography was carried out on E. Merck precoated silica gel 60 F254 plates and visualized with phosphomolybdic acid, iodine, or a UV-visible lamp.
All chemicals were purchased from Bide Pharmatech., Ltd. (Shanghai, China) and J & K scientific (Hong Kong, China). 1H-NMR and 13C-NMR spectra were collected in CDCl3 at 25 °C on a Bruker Ascend®-600 NMR spectrometer (600 MHz for 1H and 150 MHz for 13C) (Bruker, Billerica, MA, USA). All chemical shifts were reported in the standard δ notation of parts per million using the peak of residual proton signals of CDCl3 or DMSO-d6 as an internal reference (CDCl3, δC 77.2 ppm, δH 7.26 ppm; DMSO-d6, δC 39.5 ppm, δH 2.50 ppm). High-resolution mass spectra (HRMS) were measured using electrospray ionization (ESI). The measurements were done in a positive ion mode (interface capillary voltage 4500 V); the mass ratio was from m/z 50 to 3000 Da; external/internal calibration was done with electrospray calibration solution.
HRMS analyses were performed by an Agilent 6230 electrospray ionization (ESI) time-of-flight (TOF) mass spectrometer with Agilent C18 column (4.6 mm × 150 mm, 3.5 μm). The mobile phase was isocratic (water +0.01% TFA; CH3CN) at a flow rate of 0.5 mL/min. The peaks were determined at 254 nm under UV.
UV analysis was performed by a Shimadzu UV—2600 (Osaka, Japan) with 1 cm quartz cell and a slit width of 2.0 nm. The analysis was carried out using a wavelength in the range of 200–400 nm.
IR analysis (KBr) was performed by a Shimadzu IRAffinity-1S (Osaka, Japan) with a frequency range of 4000–500 cm−1.
3.1.1. Synthesis of 4-Azido-7-chloro-quinoline (2)
The 4,7-Dichloroquinoline (2.0 g, 10 mmol) was dissolved in 5 mL anhydrous DMF. NaN3 (1.3 g, 20 mmol) was then added in one portion, and the resulting mixture was stirred at 65 °C for 6 h, whereupon TLC indicated reaction completion. The reaction mixture was then allowed to cool to ambient temperature, after which it was diluted with 100 mL CH2Cl2, washed with water (3 × 30 mL), dried over anhydrous Na2SO4, and evaporated to dryness. The resulting product residue was recrystallized from a CH2Cl2/hexane 1:1 mixture to yield the final pure product 2 as colorless, needle-like crystals in 86% yield.
δH (600 MHz, CDCl3) 8.82 (1H, d, J = 4.9 Hz, H-2), 8.09 (1H, d, J = 2.4 Hz, H-8), 8.01 (1H, d, J 9.3, H-5), 7.49 (1H, dd, J 2.4 and 9.3, H-6) 7.12 (1H, d, J 4.9, H-3) ppm; δC (150 MHz, CDCl3) 150.9, 149.1, 146.8, 136.9, 127.9, 123.8, 119.9, 108.7 ppm. The spectral characteristics are consistent with those of 2 in the literature [17].
3.1.2. Synthesis of N-[4-(propargyloxy) Phenyl] acetamide (4)
Acetaminophen (N-acetyl-para-aminophenol) (13 mmol) was dissolved in 10 mL of anhydrous DMF. Anhydrous K2CO3 (2.7 g, 19.5 mmol) was then added to the solution, and the mixture was stirred at 30 °C for 30 min. Propargyl bromide (3-bromopropyne, 2.2 mL, 19.5 mmol) was then added slowly to the reaction mixture, and subsequently stirred at 30 °C for 6 h upon which TLC indicated completion of the reaction. The reaction mixture was then diluted with 50 mL water and extracted with ethyl acetate (3 × 50 mL). These extracts were then combined, washed with water (2 × 50 mL), dried over anhydrous Na2SO4 and evaporated in vacuo to yield the product residue that was then recrystallized from the CH2Cl2/hexane 1:1 mixture to yield the compound 4 in 82% yield. δH (600 MHz, CDCl3) 2.15 (3H, s, Me), 2.51 (1H, s), 4.67 (2H, s, CH2), 6.94 (2H, d, J = 8.99 Hz, HAr), 7.22 (1H, br, NH), 7.40 (2H, d, J = 8.99 Hz, HAr) ppm; δC (150MHz, CDCl3) 24.2, 56.3, 75.5, 78.5, 115.5, 121.8, 131.9, 154.6, 168.3 ppm. The spectral characteristics are consistent with those of 4 in the literature [18].
3.1.3. Synthesis of N-[7-chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl] quinoline]-acetamide (5)
The derivative of acetaminophen 4 (1mmol) and the appropriate azide 2 were dissolved in 5 mL tBuOH/water (1:1) and, while stirring at 65 °C, 1 M sodium ascorbate (0.4 mL, 0.4 mmol) and 1 M CuSO4 (0.2 mL, 20 mol%) were added sequentially, in that order. The reaction mixture was then stirred at 65 °C for 24 h. The crude product was then precipitated out by slowly adding cold water to the reaction mixture, after which it was filtered, washed with water, air dried and purified by silica column chromatography (eluents ranging in polarity from EtOAc/hex 3:7 to 5% MeOH in EtOAc). Yield 72%, δH (600 MHz, CDCl3) 2.07 (3H, s, Me), 5.33 (2H, s, CH2-O), 7.09 (2H, d, J = 9 Hz, H-15), 7.57 (2H, d, J = 9 Hz, H-16), 7.86 (1H, dd, J = 9.1 and 2.2 Hz, H-6), 7.93 (1H, d, J = 4.65 Hz, H-3), 8.03 (1H, d, J = 9.1 Hz, H-5), 8.36 (1H, d, J = 2.3 H-8), 9.01 (1H, s, H-11), 9.23 (1H, d, J = 4.65 Hz, H-2), 9.89 (1H, s, NH) ppm; δC (150MHz, CDCl3) 24.2 (C-19), 61.7 (C-13), 115.3 (C-15), 117.6 (C-3), 120.8 (C-16), 121 (C-10), 125.8 (C-5), 127.3 (C-11), 128.6 (C-8), 129.5 (C-6), 133.5 (C-17), 135.9 (C-4), 140.8 (C-7), 144.2 (C-12), 149.8 (C-9), 152.8 (C-2), 154.1 (C-14), 168.2 (CO) ppm; HRMS-ESI m/z 394.1083 [M + H]+ (calculated for C20H17ClN2O2, m/z 394.1065); UV (CH2Cl2) peaks 211, 234 and 287 nm; IR (KBr) (νmax/cm−1) 3478 (NH), 2924 (CH), 2368, 1674 (NH amide), 1612, 1550, 1512, 1458, 1411, 1373, 1319, 1242, 1049, 825 cm−1.
3.2. Biological Studies
Compound 5 was dissolved in DMSO at a final concentration of 50 mM and stored at −20 °C before use. Cytotoxicity was assessed by using the 3-(4,5-dimethylthiazole-2yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL) assay as previously described [19]. Briefly, 4 × 103 cells per well were seeded in 96-well plates before drug treatments. After overnight cell culture, the cells were then exposed to different concentrations of selected compounds (0.19–100 μM) for 72 h. Cells without drug treatment were used as controls. Subsequently, 10 μL of 5 mg/mL MTT solution was added to each well and incubated at 37 °C for 4 h followed by addition of 100 μL solubilization buffer (10 mM HCl in solution of 10% of SDS) and overnight incubation. Then A570 nm was determined in each well on the next day. The percentage of cell viability was calculated using the following formula: cell viability (%) = Atreated/Acontrol × 100. A representative graph of at least three independent experiments was shown in Supplementary Materials Figure S8.
4. Conclusions
The synthesis of a potential triazole-based quinoline was presented. The chemical structure of the synthesized compound was verified by using NMR, mass, IR and UV spectrometries. The cytotoxicity and drug likeness of the compound were also determined by MTT assay and computations, respectively.
Supplementary Materials
The following are available online, Figure S1: 1H NMR compound 4, Figure S2: 13C NMR compound 4, Figure S3: 1H NMR compound 5, Figure S4: 13C NMR compound 5, Figures S5 and S6: HSQC compound 5, Figure S7: IR spectrum compound 5, Figure S8: HRMS of 5, Figure S9: UV spectrum, Figure S10: cytotoxicity results, Table S1: 1H and 13C-nuclear magnetic spectroscopy (NMR) chemical shifts, Table S2: physicochemical properties of 5 calculated by SwissADME, Figure S11: BOILED-Egg graph.
Author Contributions
Conceptualization, P.C.; methodology, P.C. and J.P.L.N.; investigation, A.A.N.; data curation, J.P.L.N.; writing—original draft preparation, P.C.; writing—review and editing, J.P.L.N. and V.K.W.W.; supervision, P.C. and V.K.W.W.; project administration, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FDCT grants from Macao Science and Technology Development Fund to PC (project code: 0096/2020/A, 0087/2020/A).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Giovanni Ribaudo for his kind and grateful support and Yuhan Xie for support during analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinheiro, L.C.S.; Feitosa, L.M.; Gandi, M.O.; Silveira, F.F.; Boechat, N. The Development of Novel Compounds Against Malaria: Quinolines, Triazolpyridines, Pyrazolopyridines and Pyrazolopyrimidines. Molecules 2019, 24, 4095. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tsuno, N.H.; Sunami, E.; Tsurita, G.; Kawai, K.; Okaji, Y.; Nishikawa, T.; Shuno, Y.; Hongo, K.; Hiyoshi, M.; et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Danuello, A.; Bolzani, V.S.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar]
- Aher, N.G.; Pore, V.S.; Mishra, N.N.; Kumar, A.; Shukla, P.K.; Sharma, A.; Bhat, M.K. Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg. Med. Chem. Lett. 2009, 19, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Demaray, J.A.; Thuener, J.E.; Dawson, M.N.; Sucheck, S.J. Synthesis of triazole-oxazolidinones via a one-pot reaction and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2008, 18, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.P.; Yadav, A.K.; Ajay, A.; Bisht, S.S.; Chaturvedi, V.; Sinha, S.K. Application of Huisgen (3 + 2) cycloaddition reaction: Synthesis of 1-(2,3-dihydrobenzofuran-2-yl-methyl [1,2,3]-triazoles and their antitubercular evaluations. Eur. J. Med. Chem. 2010, 45, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; Brandão, G.C.; Arantes, L.M.; de Oliveira, H.A., Jr.; de Paula, R.C.; do Nascimento, M.F.; dos Santos, F.M.; da Rocha, R.K.; Lopes, J.C.; de Oliveira, A.B. 7-Chloroquinolinotriazoles: Synthesis by the azide-alkyne cycloaddition click chemistry, antimalarial activity, cytotoxicity and SAR studies. Eur. J. Med. Chem. 2014, 73, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Khan, S.I.; Rawat, D.S. Synthesis of 4-aminoquinoline-1,2,3-triazole and 4-aminoquinoline-1,2,3-triazole-1,3,5-triazine hybrids as potential antimalarial agents. Chem. Biol. Drug. Des. 2011, 78, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Boechat, N.; Ferreira, M.d.L.; Pinheiro, L.C.; Jesus, A.M.; Leite, M.M.; Júnior, C.C.; Aguiar, A.C.; de Andrade, I.M.; Krettli, A.U. New compounds hybrids 1h-1,2,3-triazole-quinoline against Plasmodium falciparum. Chem. Biol. Drug Des. 2014, 84, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Singh, P.; Singh, P.; Gut, J.; Rosenthal, P.J.; Kumar, V. Azide-alkyne cycloaddition en route to 1H-1,2,3-triazole-tethered 7-chloroquinoline-isatin chimeras: Synthesis and antimalarial evaluation. Eur. J. Med. Chem. 2013, 62, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Melato, S.; Coghi, P.; Basilico, N.; Prosperi, D.; Monti, D. Novel 4-Aminoquinolines through Microwave-Assisted SNAr Reactions: A Practical Route to Antimalarial Agents. Eur. J. Org. Chem. 2007, 36, 6118–6123. [Google Scholar] [CrossRef]
- De Souza, M.V.; Pais, K.C.; Kaiser, C.R.; Peralta, M.A.; de L Ferreira, M.; Lourenço, M.C. Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. Bioorg. Med. Chem. 2009, 174, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.K.; Colasson, B.; Fokin, V.V. One-Pot Synthesis of 1,4-Disubstitued 1,2,3-Triazole from In Situ Generated Azides. Org. Lett. 2004, 6, 3897–3899. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hongbin, Y.; Chaofeng, L.; Lixia, S.; Jie, L.; Yingchun, C.; Zhuang, W.; Weihua, L.; Guixia, L.; Yun, T. AdmetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2018, 35, 1067–1069. [Google Scholar]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, synthesis, and in vitro antimalarial evaluation of triazole linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem. 2010, 18, 8243–8256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, K.; Lan, S.; Zhang, L. Rapid access to chroman-3-ones through gold-catalyzed oxidation of propargyl aryl ethers. Angew. Chem. Int. Ed. 2012, 51, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.K.; Coghi, P.; Agarwal, P.; Shyamlal, R.B.K.; Yadav, L.; Sharma, R.; Yadav, D.K.; Sahal, D.; Wong, V.K.W.; Chaudhary, S. Novel functionalized 1,2,4-Trioxanes as Potent Antimalarial and Anticancer Agents: Design, Synthesis, Structure Activity Relationship and in silico docking studies. Chem. Med. Chem. 2020, 15, 1216. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).