N-[7-Chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide
Abstract
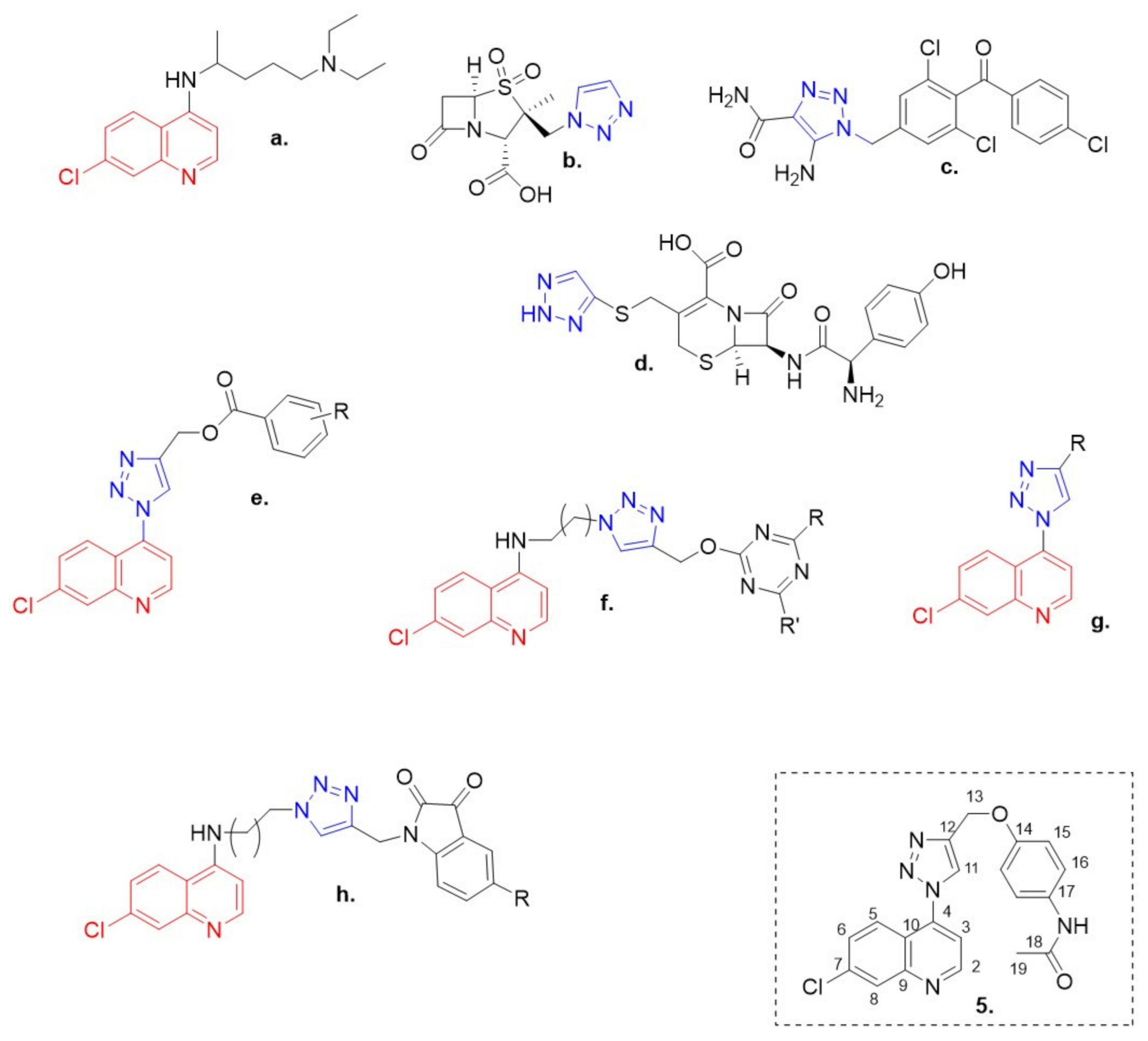
1. Introduction
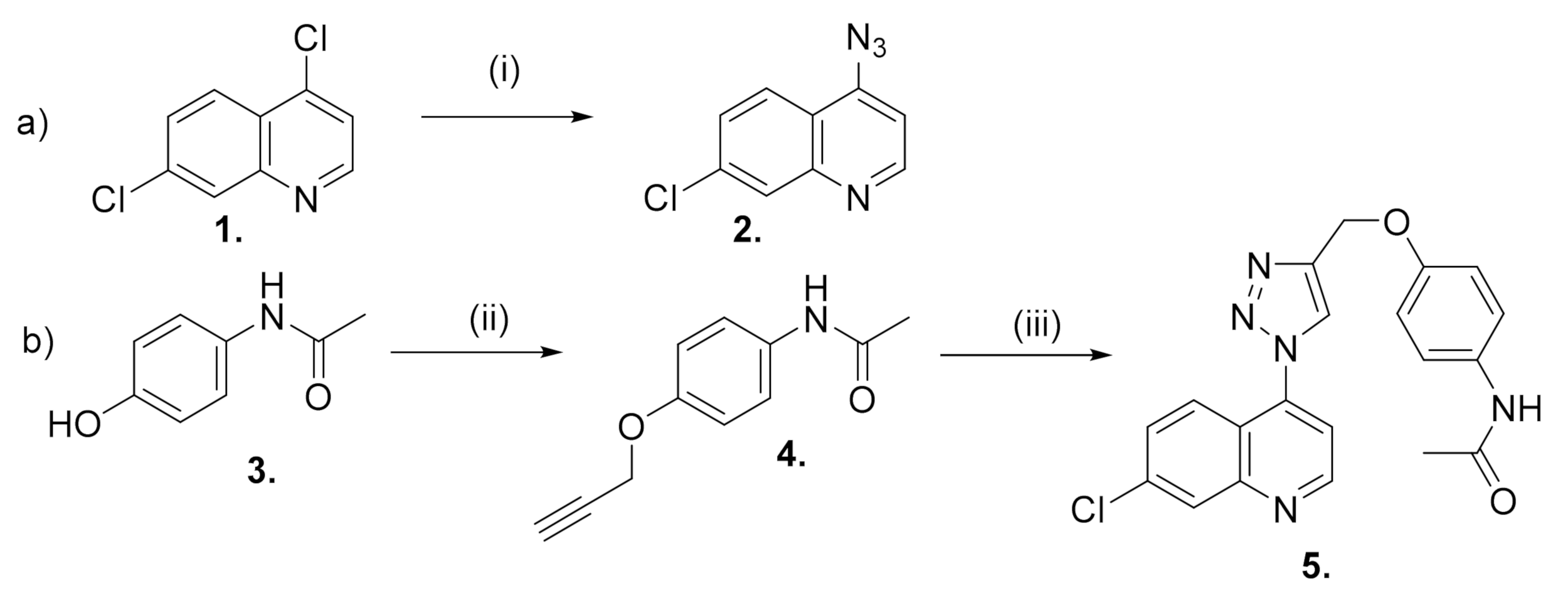
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 4-Azido-7-chloro-quinoline (2)
3.1.2. Synthesis of N-[4-(propargyloxy) Phenyl] acetamide (4)
3.1.3. Synthesis of N-[7-chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl] quinoline]-acetamide (5)
3.2. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinheiro, L.C.S.; Feitosa, L.M.; Gandi, M.O.; Silveira, F.F.; Boechat, N. The Development of Novel Compounds Against Malaria: Quinolines, Triazolpyridines, Pyrazolopyridines and Pyrazolopyrimidines. Molecules 2019, 24, 4095. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tsuno, N.H.; Sunami, E.; Tsurita, G.; Kawai, K.; Okaji, Y.; Nishikawa, T.; Shuno, Y.; Hongo, K.; Hiyoshi, M.; et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Danuello, A.; Bolzani, V.S.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar]
- Aher, N.G.; Pore, V.S.; Mishra, N.N.; Kumar, A.; Shukla, P.K.; Sharma, A.; Bhat, M.K. Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg. Med. Chem. Lett. 2009, 19, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Demaray, J.A.; Thuener, J.E.; Dawson, M.N.; Sucheck, S.J. Synthesis of triazole-oxazolidinones via a one-pot reaction and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2008, 18, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.P.; Yadav, A.K.; Ajay, A.; Bisht, S.S.; Chaturvedi, V.; Sinha, S.K. Application of Huisgen (3 + 2) cycloaddition reaction: Synthesis of 1-(2,3-dihydrobenzofuran-2-yl-methyl [1,2,3]-triazoles and their antitubercular evaluations. Eur. J. Med. Chem. 2010, 45, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.R.; Brandão, G.C.; Arantes, L.M.; de Oliveira, H.A., Jr.; de Paula, R.C.; do Nascimento, M.F.; dos Santos, F.M.; da Rocha, R.K.; Lopes, J.C.; de Oliveira, A.B. 7-Chloroquinolinotriazoles: Synthesis by the azide-alkyne cycloaddition click chemistry, antimalarial activity, cytotoxicity and SAR studies. Eur. J. Med. Chem. 2014, 73, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Khan, S.I.; Rawat, D.S. Synthesis of 4-aminoquinoline-1,2,3-triazole and 4-aminoquinoline-1,2,3-triazole-1,3,5-triazine hybrids as potential antimalarial agents. Chem. Biol. Drug. Des. 2011, 78, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Boechat, N.; Ferreira, M.d.L.; Pinheiro, L.C.; Jesus, A.M.; Leite, M.M.; Júnior, C.C.; Aguiar, A.C.; de Andrade, I.M.; Krettli, A.U. New compounds hybrids 1h-1,2,3-triazole-quinoline against Plasmodium falciparum. Chem. Biol. Drug Des. 2014, 84, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Singh, P.; Singh, P.; Gut, J.; Rosenthal, P.J.; Kumar, V. Azide-alkyne cycloaddition en route to 1H-1,2,3-triazole-tethered 7-chloroquinoline-isatin chimeras: Synthesis and antimalarial evaluation. Eur. J. Med. Chem. 2013, 62, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Melato, S.; Coghi, P.; Basilico, N.; Prosperi, D.; Monti, D. Novel 4-Aminoquinolines through Microwave-Assisted SNAr Reactions: A Practical Route to Antimalarial Agents. Eur. J. Org. Chem. 2007, 36, 6118–6123. [Google Scholar] [CrossRef]
- De Souza, M.V.; Pais, K.C.; Kaiser, C.R.; Peralta, M.A.; de L Ferreira, M.; Lourenço, M.C. Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. Bioorg. Med. Chem. 2009, 174, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.K.; Colasson, B.; Fokin, V.V. One-Pot Synthesis of 1,4-Disubstitued 1,2,3-Triazole from In Situ Generated Azides. Org. Lett. 2004, 6, 3897–3899. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hongbin, Y.; Chaofeng, L.; Lixia, S.; Jie, L.; Yingchun, C.; Zhuang, W.; Weihua, L.; Guixia, L.; Yun, T. AdmetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2018, 35, 1067–1069. [Google Scholar]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, synthesis, and in vitro antimalarial evaluation of triazole linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem. 2010, 18, 8243–8256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, K.; Lan, S.; Zhang, L. Rapid access to chroman-3-ones through gold-catalyzed oxidation of propargyl aryl ethers. Angew. Chem. Int. Ed. 2012, 51, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.K.; Coghi, P.; Agarwal, P.; Shyamlal, R.B.K.; Yadav, L.; Sharma, R.; Yadav, D.K.; Sahal, D.; Wong, V.K.W.; Chaudhary, S. Novel functionalized 1,2,4-Trioxanes as Potent Antimalarial and Anticancer Agents: Design, Synthesis, Structure Activity Relationship and in silico docking studies. Chem. Med. Chem. 2020, 15, 1216. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coghi, P.; Ng, J.P.L.; Nasim, A.A.; Wong, V.K.W. N-[7-Chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide. Molbank 2021, 2021, M1213. https://doi.org/10.3390/M1213
Coghi P, Ng JPL, Nasim AA, Wong VKW. N-[7-Chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide. Molbank. 2021; 2021(2):M1213. https://doi.org/10.3390/M1213
Chicago/Turabian StyleCoghi, Paolo, Jerome P. L. Ng, Ali Adnan Nasim, and Vincent Kam Wai Wong. 2021. "N-[7-Chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide" Molbank 2021, no. 2: M1213. https://doi.org/10.3390/M1213
APA StyleCoghi, P., Ng, J. P. L., Nasim, A. A., & Wong, V. K. W. (2021). N-[7-Chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide. Molbank, 2021(2), M1213. https://doi.org/10.3390/M1213







